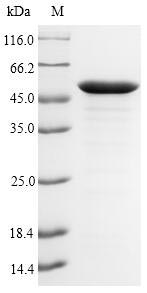
Recombinant Pseudomonas aeruginosa UDP-3-O-acyl-N-acetylglucosamine deacetylase (lpxC) gets expressed in E. coli and contains the complete 1-303 amino acid sequence. The protein carries an N-terminal 6xHis-SUMO tag, which appears to improve both solubility and purification efficiency. SDS-PAGE analysis indicates purity levels above 90%, suggesting this preparation should deliver reliable results in research settings. This product is intended strictly for research applications and may prove useful for detailed studies of bacterial biochemistry.
UDP-3-O-acyl-N-acetylglucosamine deacetylase—better known as lpxC—seems to be critical for lipid A biosynthesis, an important part of bacterial outer membrane structure. The enzyme handles the deacetylation step, which is likely essential for producing lipid A precursors. Getting a handle on lpxC activity could be valuable for research into how bacteria build their cell walls. This knowledge might also help in creating new approaches to combat bacterial infections.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Pseudomonas aeruginosa LpxC is a zinc-dependent metalloenzyme that requires precise folding, proper active site formation with zinc ion coordination, and specific tertiary structure for its deacetylase activity in lipid A biosynthesis. The E. coli expression system is homologous to this bacterial enzyme, which increases the probability of correct folding and metal incorporation. The N-terminal 6xHis-SUMO tag (∼15 kDa) may cause some steric interference but is less problematic for this full-length protein (303aa, ∼34 kDa). While the homologous expression system favors proper folding, the probability of correct folding with functional enzymatic activity requires experimental validation of zinc incorporation and catalytic activity.
1. Biochemical Characterization and Enzyme Kinetics Studies
This application carries a significant risk without functional validation. LpxC enzymatic activity requires precise zinc coordination and active site formation. If correctly folded and active (verified through deacetylase assays), the protein may be suitable for kinetic studies. If misfolded/inactive (unverified), kinetic measurements will yield biologically meaningless results. The SUMO tag may sterically interfere with substrate access.
2. Antibody Development and Immunological Studies
This application is highly suitable as antibody development relies on antigenic sequence recognition rather than functional enzymatic activity. The full-length protein provides comprehensive epitope coverage for generating LpxC-specific antibodies. The high purity (>90%) ensures minimal contamination-related issues during immunization protocols.
3. Protein-Protein Interaction Studies
This application requires proper folding validation. LpxC interactions with regulatory proteins require precise tertiary structure. If correctly folded (verified), the protein may identify physiological interaction partners. If misfolded/unverified, there is a risk of non-specific binding or failure to replicate genuine protein interactions.
4. Inhibitor Screening and Drug Discovery Research
This application carries a high risk without functional validation. Inhibitor screening requires native enzyme conformation and catalytic activity. If correctly folded and active (verified), limited screening may be possible. If misfolded/inactive (unverified), screening results will be unreliable for drug discovery.
Final Recommendation & Action Plan
The E. coli expression system is favorable for producing this bacterial LpxC due to homologous expression conditions, but the large SUMO tag necessitates experimental validation before functional applications. Begin with zinc content analysis and enzymatic activity assays using UDP-3-O-acyl-GlcNAc substrate to confirm functionality. Applications 1, 3, and 4 require rigorous functional validation before proceeding. Application 2 (antibody development) can proceed immediately. For reliable LpxC research, consider SUMO tag removal for critical kinetic and inhibitor studies to minimize potential steric interference with the active site.