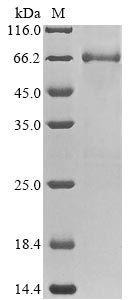
Recombinant Rat Electron transfer flavoprotein-ubiquinone oxidoreductase (Etfdh) gets expressed in E. coli and covers the full mature protein region from amino acids 33 to 616. The protein carries an N-terminal 6xHis-tag, which makes purification and detection much easier. SDS-PAGE analysis confirms a purity level greater than 85%, suggesting it should work well for different research applications. This product is meant for research use only.
Electron transfer flavoprotein-ubiquinone oxidoreductase (Etfdh) appears to play a crucial role in mitochondrial electron transport. It participates in transferring electrons from electron transfer flavoprotein to the ubiquinone pool. This protein is vital in cellular energy metabolism since it contributes to oxidative phosphorylation. Understanding how it works may be important for studying metabolic pathways and mitochondrial disorders.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Etfdh is a flavoprotein that normally localizes to mitochondria, requiring proper FAD cofactor incorporation, iron-sulfur cluster assembly, and membrane-associated folding—processes that depend heavily on eukaryotic mitochondrial chaperones and redox systems. The E. coli cytosolic environment generally lacks the necessary machinery for correct cofactor loading and folding of mitochondrial oxidoreductases, and the protein may form misfolded or partially inactive aggregates even when expressed as the mature form (excluding the mitochondrial signal peptide). Therefore, although soluble expression may occur, the recombinant Etfdh is unlikely to be functionally active or properly folded in a way that replicates the native mitochondrial enzyme. However, the protein may retain partial structure and linear epitopes, making it still suitable for antibody production and limited structural or immunochemical assays.
1. Biochemical Characterization of Mitochondrial Electron Transport Chain Components
This recombinant rat Etfdh protein can be used for preliminary biochemical characterization, such as assessing solubility, secondary structure, or cofactor-binding potential. However, since it was produced in E. coli, it is unlikely to be correctly folded or enzymatically active, as proper assembly of redox cofactors (FAD and Fe-S clusters) and mitochondrial folding conditions are missing. If experimental confirmation of enzymatic function has not been performed, studies should be treated as non-functional structural analyses rather than true kinetic investigations.
2. Antibody Development and Validation
The recombinant Etfdh protein is suitable as an antigen for generating antibodies against the rat enzyme, particularly those recognizing linear epitopes or denatured forms. While it may not represent the native conformation, its full-length mature sequence (33–616aa) provides extensive epitope coverage. Resulting antibodies should be validated in rat tissue lysates or mitochondria-enriched samples to confirm recognition of the native protein.
3. Comparative Protein Structure and Function Analysis
This E. coli-expressed Etfdh may be used for basic comparative studies, such as thermal stability or sequence-related property evaluation, but functional or cofactor-binding comparisons with eukaryotic homologs are not reliable unless refolding or cofactor reconstitution is performed. Thus, it serves best as a reference for structural domain mapping, not as a proxy for in vivo functional characterization.
Final Recommendation & Action Plan
The E. coli-expressed recombinant rat Etfdh (33–616aa) is unlikely to be enzymatically active or correctly folded, since the protein requires mitochondrial cofactor assembly systems and oxidative folding pathways absent in bacteria. It can, however, be used effectively as an antigen for antibody production, in limited biophysical assays, or for epitope mapping. Avoid direct kinetic or cofactor-binding studies unless protein refolding and FAD/Fe-S reconstitution are experimentally verified.