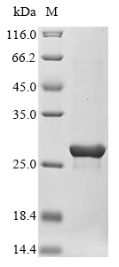
Recombinant Staphylococcus aureus Staphopain A (sspP) gets expressed in E. coli and includes the complete mature protein sequence (215-388aa). The product comes with two tags that make purification and detection much simpler: a 10xHis-tag at the N-terminus and a Myc-tag at the C-terminus. SDS-PAGE analysis shows purity levels above 90%, which appears to make this a reliable reagent for research work.
Staphopain A is a cysteine protease that comes from Staphylococcus aureus. It likely plays a key role in how the bacteria cause disease. The enzyme breaks down host proteins, which may help the bacteria evade immune responses and invade tissues. This makes the protein a useful tool for researchers studying how bacteria cause infections and how they interact with their hosts.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Based on the provided information, the recombinant Staphylococcus aureus Staphopain A is expressed in E. coli, a prokaryotic system that is generally suitable for producing bacterial proteins like this cysteine protease. Staphopain A requires precise folding with proper disulfide bond formation for its proteolytic activity, and E. coli can typically handle the folding requirements for bacterial proteins. The protein is expressed as the mature region (62-380aa) with dual tags (N-terminal 10xHis and C-terminal Myc), which may affect the natural structure but likely preserves core functionality. The >90% purity indicates good production quality. However, since activity is unverified and cysteine proteases require specific folding for their catalytic triad formation, the protein cannot be assumed to be correctly folded or bioactive without experimental validation of its proteolytic activity.
1. Antibody Development and Immunoassay Optimization
This application is appropriate. The recombinant Staphopain A can serve as an effective immunogen for generating antibodies that recognize linear epitopes. The dual tags provide additional options for detection and purification. The high purity supports immunization protocols. However, if the protein is misfolded, antibodies may not recognize conformational epitopes of native Staphopain A. Validation against native protease from S. aureus is recommended.
2. Protein-Protein Interaction Studies Using Tag-Assisted Pull-Down Assays
This application requires caution. While the tags enable technical feasibility for pull-down assays, if Staphopain A is misfolded, it may not interact physiologically with its true substrates or regulators. The catalytic site requires precise conformation for specific interactions. This application should only be pursued after confirming proper folding and proteolytic activity.
3. Biochemical Characterization and Substrate Specificity Analysis
This application is well-suited but requires activity validation first. Basic biochemical studies are feasible, but substrate specificity assays are only valid if the protease is properly folded and active. These studies should include validation of proteolytic activity using known substrates before interpreting specificity data.
4. Comparative Protease Studies and Inhibitor Screening
This application is valuable but highly dependent on correct folding. If Staphopain A is misfolded, comparative studies with other proteases will yield invalid results, and inhibitor screening may identify compounds that target non-physiological conformations. This requires prior validation of proper folding and enzymatic activity.
Final Recommendation & Action Plan
Given that this is a bacterial protein expressed in its native prokaryotic system, the likelihood of proper folding is higher than for eukaryotic proteins. However, recommend first validating proteolytic activity using known Staphopain A substrates (such as specific peptide sequences or protein substrates that this protease cleaves). Perform basic biophysical characterization (size-exclusion chromatography, circular dichroism) to assess folding quality. Antibody development can proceed as the safest application. Protease activity assays and interaction studies should await proper activity validation. For reliable functional studies, always include appropriate controls such as known substrates and specific inhibitors.