Apoptosis mediated by death receptor
1. Apoptosis instruction
2. Pathways
2.1 Endogenous mitochondrial pathway
2.2 Endogenous endoplasmic reticulum pathway
2.3 Exogenous death receptor pathway
Death
receptor (DR) is a member of tumor necrosis factor receptor (TNFR) superfamily
with extracellular Cys-rich domain and intracellular death domain (DD). When
the death receptor combines with specific death ligand, it receives
extracellular death signal, and activates the mechanism of apoptosis in cells,
inducing apoptosis. The main death receptors- ligands known to date are Fas (APO-1, CD95)- FasL (CD95L),
TNFR1 (DR1)- TNF, TRAILR1 (DR4)- TRAIL (APO-2L), TRAILR2 (DR5)- TRAIL (APO-2L),
DR3 (APO-3, TRAMP)- TL1A and so on. Currently, there are 3 major apoptotic
death receptor signaling pathways: Fas, TNFR1, TRAIL.
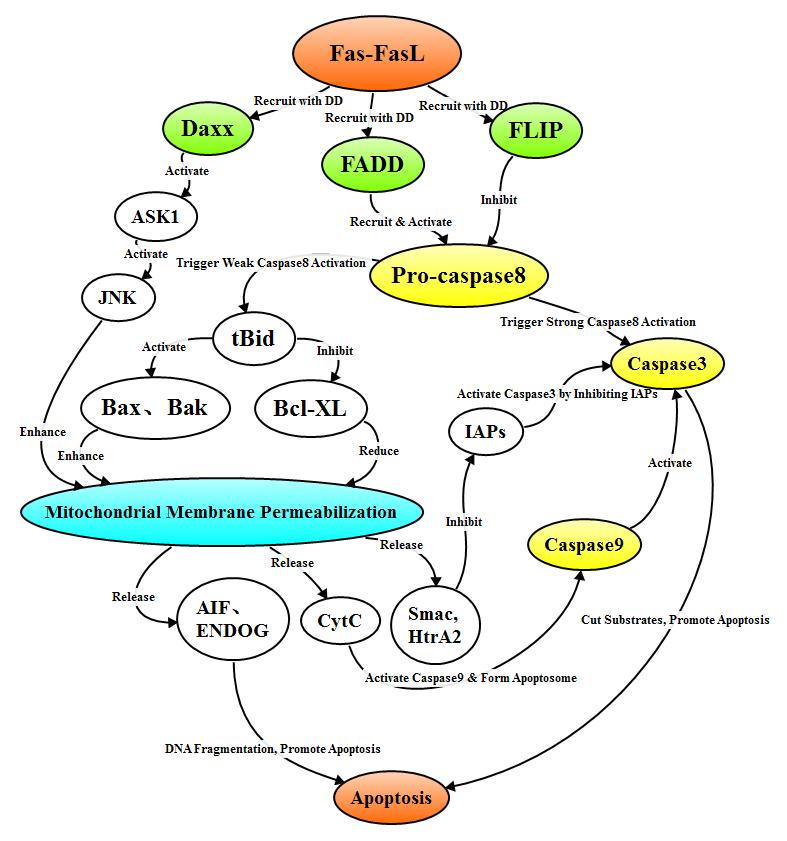
2.3.1 Fas signal pathway
When
the FasL homotrimer complex binds to Fas, it initiates Fas-FasL- mediated
apoptosis of the external death receptor pathway. In the process of signal
transduction, the 3 Fas receptor molecules combine with ligand to form trimer
and clustered intracellular DD recruits FADD, Daxx, FAP-1, FLIP and other
related proteins. FADD recruits pro-caspase 8 through death effector domain DED
to form death-inducing signaling complex (DISC), and pro-caspase8 in DISC
self-cleaves into active Caspase8. FLIP contains DED which allows it to be
integrated into the DISC of death receptor, FLIP in turn inhibits pro-caspase 8
activation by competitively binding DED on FADD or DED on Caspase8.
The
pathways by which protein caspase8 activiates Caspase3 varies in different
types of cells. In type I cells, the DISC complex activates a large number of
Caspase8, Caspase8 activates Caspase3, and Caspase3 acts on various substrates
that cause apoptosis, and lead to apoptosis finally; In type II cells, only a
small amount of Caspase8 is activated. Actived Caspase8 can stimulate the
transformation of Bid into tBid which is then recloated to the mitochondrial
membrane, activitng the Bcl-2 family pro-apoptotic factors and inhibits the Bcl-2
family anti-apoptotic factors, which mediates apoptosis through the
mitochondrial pathway. In addition, Daxx recruited by the DD domain of Fas can
also activate JNK signaling pathway, and enhance the transcriptional expression
of pro-apoptotic genes such as p53, Fas and FasL through mitochondrial pathway
to mediate apoptosis.
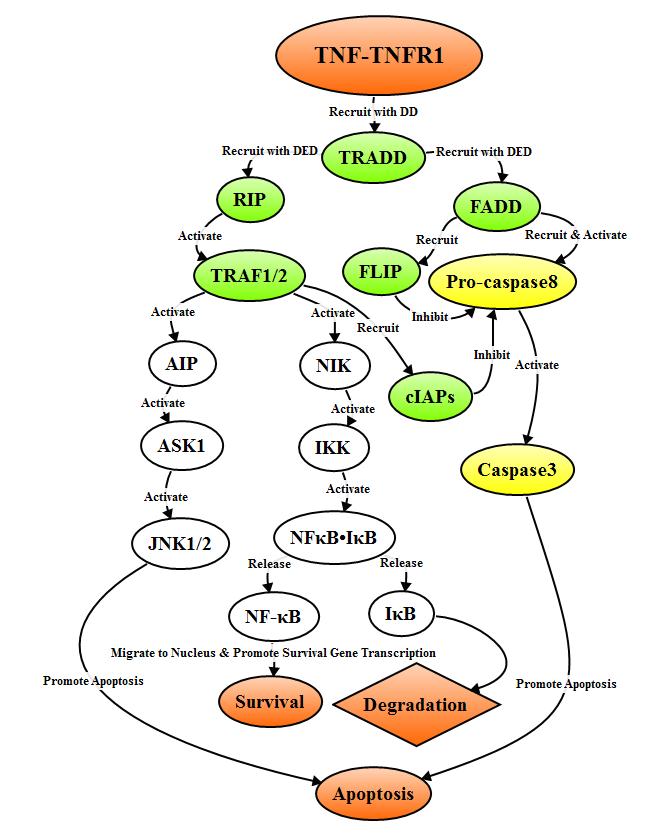
2.3.2 TNFR1 signal pathway
TNF
trimer binds to TNFR1 to induce the clustering of DD of TNFR1 to recruit adapter
protein TRADD which recruits signaling molecules such as TRAF2, RIP and FADD. TRAF2
and RIP can activate signal pathways of NF-κB and JNK/AP, while FADD triggers
caspase cascade, the survival of cells depends on the different adapter
molecules recruited by TRADD.
FADD recruits and activates pro-caspase8
through DED to form active Caspase8 after binding to adapter protein TRADD.
Caspase8 triggers Caspase cascade to mediate apoptosis, while FADD also can
recruit FLIP to inhibit the release of active Caspase8. When the adapter
protein TRADD binds to RIP by DD, it activates the TNFR related factor (TRAF-2)
which binds to TRAF-1 and recruit cIAPs, the formed complex inhibits the
activity and release of Caspase8, and thereby inhibiting apoptosis. In
addition, TRAF-2 can activate NF-κB-inducing kinase (NIK), which in turn
activates IκB kinase (IKK) through phosphorylation. IKK phosphorylates IκB and releases
NF-κB, then translocates to the nucleus and activates anti-apoptotic genes such
as cIAP, FLIP, Bcl-XL expression, and promote cell survival.
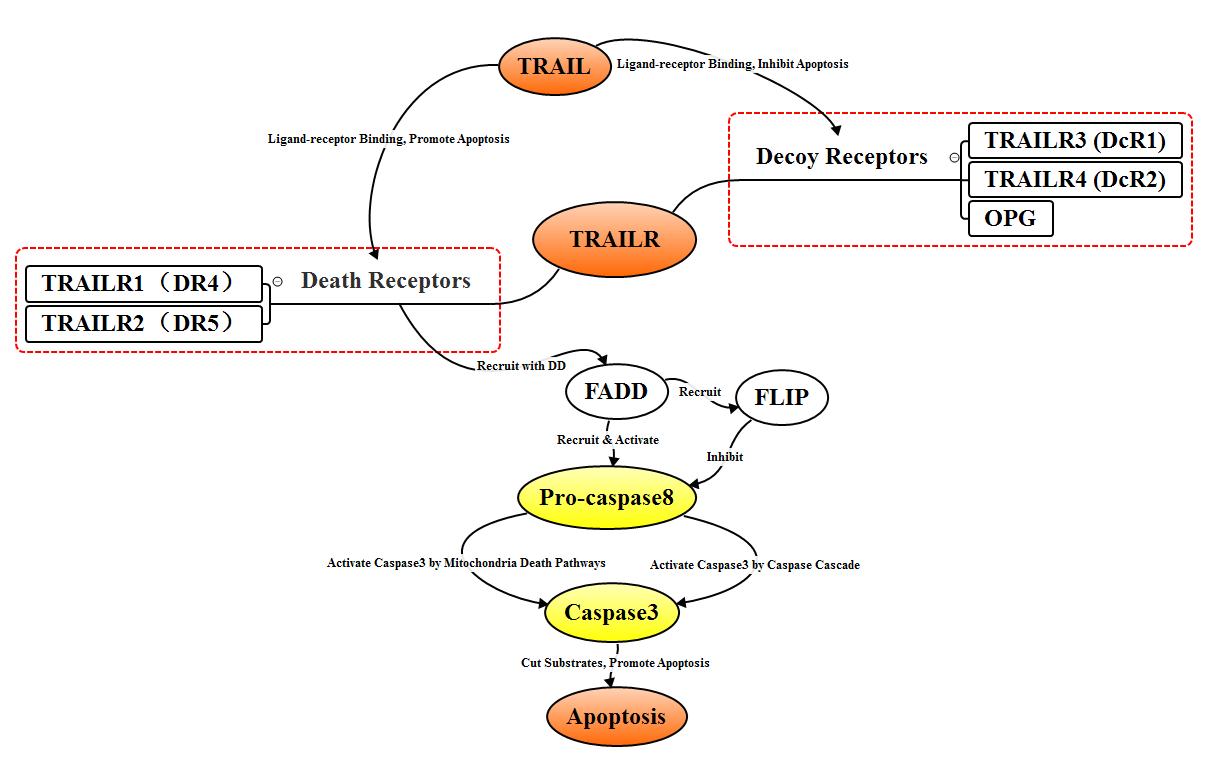
2.3.3 TRAIL signal pathway
TNF related apoptosis-inducing ligand (TRAIL)
are type II transmembrane proteins. To date, at least 5 TRAIL receptors TRAILR1
(DR4), TRAILR2 (DR5), TRAILR3 (DcR1), TRAILR4 (DcR2), OPG have been found. TRAIL
receptors can be divided into two categories, one is the decoy receptors like
TRAILR3, TRAILR4, OPG, and the other one is the death receptors like TRAILR1,
TRAILR2. Inducible receptors are mainly found in normal cells. When combined
with ligand TRAIL, they can form to non-functional complexes and block
apoptosis. The expression of TRAILR1 and TRAILR2 was significantly increased in
cancer cells after binding with ligand TRAIL, pro-caspase8 was recruited by the
combination of DD and FADD to form DISC, pro-caspase8 in DISC self-cleaves into
active Caspase8 which mediates apoptosis by activating Caspase3 through a
Caspase pathway similar to Fas and a mitochondria-dependent pathway.
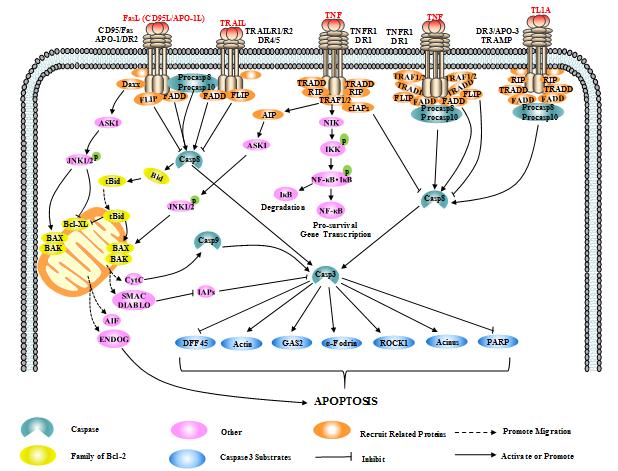
Signal pathways of death receptors mediate apoptosis
Click for more apoptotic antibodies
Past review
Apoptosisi mediated by mitochondria
Apoptosisi mediated by endoplasmic reticulum
The next notice
Picture post
3. Cited references
[1] Declercq W,
Vanden B T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival [J]. Cell, 2009, 138(2):
229.
[2] Kantari C,
Walczak H. Caspase-8 and bid: caught in the act between death receptors and
mitochondria [J]. Biochimica et Biophysica Acta, 2011, 1813(4): 558-563.
[3] Kaufmann T,
Strasser A, Jost P J. Fas death receptor signalling: roles of Bid and XIAP [J].
Cell Death & Differentiation, 2012, 19(1): 42-50.
[4] Lavrik I N,
Krammer P H. Regulation of CD95/Fas signaling at the DISC [J]. Cell Death &
Differentiation, 2012, 19(1): 36-41.
[5] Van H F,
Festjens N, Declercq W, et al. Tumor necrosis factor-mediated cell death: to
break or to burst, that's the question [J]. Cellular and
Molecular Life Sciences, 2010, 67(10): 1567-1579.
[6] Wajant H,
Scheurich P. TNFR1-induced activation of the classical NF-κB pathway [J]. The
FEBS Journal, 2015, 278(6): 862-876.
[7] Yoon J H,
Gores G J. Death receptor-mediated apoptosis and the liver [J]. Journal of
Hepatology, 2002, 37(3): 400-410.
[8] Wajant H.
Death receptors [J]. Essays in Biochemistry, 2003, 39: 53-71.
[9] Lavrik I,
Golks A, Krammer P H. Death receptor signaling [J]. Journal of Cell Science,
2005, 118(2): 265-267.
[10] Sartorius U, Schmitz I, Krammer P H. Molecular
mechanisms of death-receptor-mediated apoptosis [J]. Chembiochem: A European
Journal of Chemical Biology, 2001, 2(1): 20-29.
[11] Schmitz I, Kirchhoff S, Krammer P H. Regulation
of death receptor-mediated apoptosis pathways [J]. International Journal of
Biochemistry & Cell Biology, 2000, 32(11-12): 1123-1136.
Cite this article
CUSABIO team. Apoptosis mediated by death receptor. https://www.cusabio.com/c-20473.html






Comments
Leave a Comment