On January 7, 2021, a study published in the journal Cancer Research showed that TNFR1-mediated apoptosis and necrosis are associated with leukemia [1]. TNFR1, as an emerging biological target, numerous studies confirm that TNFR1 has either a pro-death or pro-survival role in cancer, as well as involved in autoimmune diseases. More intriguingly, data from studies have implied that TNFR1 inhibitors are expected to be candidates for the treatment of COVID-19. Nevertheless, turning this potential into practice faces challenges due to the complex biological functions of TNFR1. Back to September 2020, therapeutic antibody pioneers Professor Sir Marc Feldmann and Dr. H. Michael Shepard, as world-renowned scholars, launched a project to develop specific TNFR1 inhibitors that block TNFR1 for the treatment of acute inflammation and multiple cancers. So what is TNFR1? What signaling pathway does TNFR1 mediate? How does TNFR1 affect COVID-19? What is the potential value of TNFR1-targeted treatment?
1. TNFR1 Structure and Function
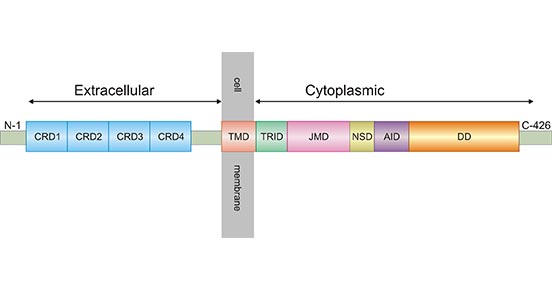
TNFR1 (as known as TNFRSF1A, CD120a or p55), a member of the tumor necrosis factor (TNF) receptor superfamily, is a type I transmembrane glycoprotein with a molecular weight of 55 ku [2]. TNFR1 has extracellular, transmembrane and intracellular domains. The extracellular region is approximately 182 amino acids long and consists of four cysteine rich domains (CRD1-CRD2-CRD3-CRD4) starting from the N-terminus. The transmembrane region lies between the extracellular and intracellular domains and it is approximately 22 amino acids long (residues 183-205). The intracellular/cytoplasmic domain of TNFR1 is about 223 amino acids long and is important for activation of several downstream signaling pathways via its conserved death domain (DD). In resting cells, the silencer of death domain (SODD) binds and inhibits the function of death domain (Figure 1) [3, 4].
Figure 1. Schematic diagram for TNFR1 structure
*The figure is derived from ResearchGate publication [4]
TNFR1 is widely distributed on the surfaces of various immune system-related cell types such as macrophages and regulatory T cells as well as in many tumor cells [5]. TNFR1 mainly mediates apoptotic signaling pathway, leading to apoptosis, which plays an important role in anti-tumor and anti-viral. It is also involved in autoimmune diseases, being a key factor in the induction of rheumatoid arthritis (RA) [6] and systemic lupus erythematosus (SLE) [7]. TNFR1, a double-edged sword, its underlying molecular mechanism still remains unclear.
2. The Ligand of TNFR1
TNF is a compact trimer composed of three identical subunits [8]. TNF contains TNF-α and TNF-β, which are type II membrane proteins. TNF-α is mostly produced by activated macrophages, whereas TNF-β is produced by activated lymphocytes [9] Currently, TNF is often called TNF-α since the TNF-β level has been shown to be quite less than TNF-α [10].
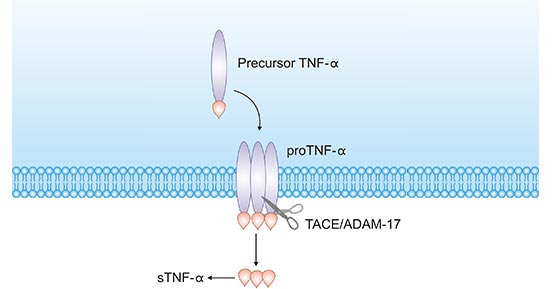
TNFα exists in two forms, transmembrane TNF-α (tmTNF-α) and soluble TNF-α (sTNF-α). tmTNF-α is the precursor form of sTNF-α. After processing by TACE-converting enzyme (also known as ADAM17/CD156q), sTNF-α is generated (Figure 2) [11].
Figure 2. tmTNF-α to sTNF-α process
*The figure is derived from the Ochsner Journal publication [11]
Both types of TNFα; interact with receptors (TNFR1 or TNFR2) to induce biological effects, but since TNFR2 lacks a death domain, TNFR1 becomes the primary receptor mediating TNF-α; activity [12, 13, 14]. Binding of TNFR1 to TNF-α; causes changes in the conformation of the intracellular region, which attributes to the TNFR1 activation [15].
3. TNFR1-Mediated Signaling Pathways
3.1 TNFR1-sTNF-α Signaling Pathway
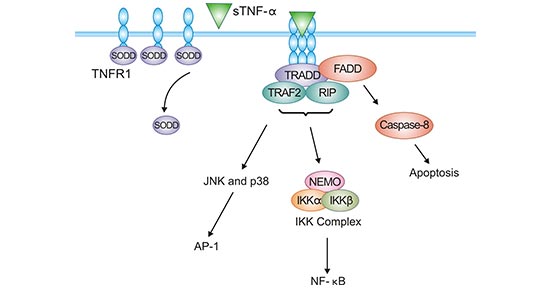
Regarding the TNFR1-sTNF-α signaling pathway, it has been well-defined in recent years. Without sTNF-α stimulation, the DD of TNFR1 is occupied by the silencer of the death domain (SODD), which blocks the TNFR-related death domain protein (TRADD) from combing with TNFR1. TNFR1 signaling pathway is restrained [16]. Upon sTNF-α stimulation, SODD dissociates from the TNFR1 DD. TNFR1 recruits TRADD and the TNFR1 signaling pathway is activated (Figure 3) [17].
Once TNFR1/sTNF-α is activated, TRADD can recruit receptor-associated protein-1 (RIP1) and receptor-associated factor 2 (TRAF2), which activates NF-κB. The activation of NF-κB promotes target gene transcription, which resists apoptosis and promotes survival (Figure 3) [4, 17]
In addition, TNFR1 binds to DD of FADD (Fas-associated death domain protein) through DD of TRADD. FADD can bind to DED of caspase-8 through its death-effective DED domain, which activates caspase-8, leading to apoptosis or programmed necrosis (necroptosis) (Figure 3) [4, 17].
Figure 3. TNFR1-sTNF-α signaling pathway
*The figure is derived from Cytokine publication [17]
3.2 TNFR1-tmTNF-α Signaling Pathway
Regarding the signaling pathway of TNFR1-tmTNF-α, the current mechanism is unclear. Some studies have found that the TNFR1-tmTNF-α signaling pathway is different from TNFR1-sTNF-α, which can mediate two distinct signaling pathways, survival or apoptosis. While TNFR1-tmTNF-α only mediates apoptosis and does not activate NF-κB. Although TNFR1 binding to both types of TNF can cause apoptosis, they have different signaling mechanisms and different cellular localization [4].
4. The Role of TNFR1 in Tumors
In recent years, more and more researchers have found that TNFR1 is widely involved in the pathophysiological processes of various diseases, especially in tumors. Thus its regulatory role has attracted attention. Extensive data have shown that TNFR1 can determine different cell fates in different cancer cells. Although TNFR1 contains the death domain, TNFR1 can also exert pro-inflammatory and oncogenic effects through different signaling pathways. Therefore, TNFR1 does not always exert pro-apoptotic biological effects in cancer.
In malignant astrocytomas, TNFR1 binds to TNF, activates the NF-κB pathway, and inhibits apoptosis of tumor cells. The study suggests that TNFR1 may be involved in the formation of low-grade malignant astrocytomas [18]. Another study found that in gastric cancer cells, the expression level of TNFR1 was correlated with the degree of differentiation of gastric cancer cells [19]; in colorectal cancer, patients with high TNFR1 expression had a higher survival rate [20]. A recent study found that in renal clear cell carcinoma, TNFR1 promotes tumor-like lesions and induces drug resistance [21]. In a mouse model experiment, researchers evaluated the role of TNFR1 in hepatocellular carcinoma. The data showed that TNFR1 deficiency significantly reduced tumorigenesis in mice, suggesting that TNFR1-mediated signaling pathways promote hepatocarcinogenesis [22].
TNFR1 is involved in an extremely complex biological process, and its functions is often influenced by many uncontrollable factors (e.g., individualized patient differences). The specified roles of TNFR1 in human tumors are not well understood. Whether it mediates cell proliferation or apoptosis in different tumors requires more studies to further elucidate. Therefore, the application of TNFR1 antibodies or anti-TNFR1 antibodies is of great significance in tumor therapy.
5. Potential Value of TNFR1 in the Treatment of COVID-19
Since the COVID-19 pandemic, COVID-19 has been identified in almost every country on the planet, killing more than 2 million people worldwide. Notably, in COVID-19 patients, researchers have found high levels of inflammatory and pro-inflammatory cytokines, including TNF. As mentioned earlier, the function of TNF is mediated primarily through TNFR1, so blocking TNFR1 alone to maintain immune system function has become the most desirable way for researchers. Although there are currently no antibody drugs on the market that inhibit TNFR1, it is exciting to note that an anti-TNFR1 drug (GSK-2862277) developed by GlaxoSmithKline (GSK) has entered clinical phase II for the treatment of acute lung injury.
In fact, the pharmaceutical industry and academia are making unprecedented efforts to fight COVID-19. Global companies such as Johnson & Johnson, Sanofi, Pfizer, Merck and others are developing nearly 200 therapeutic agents, and governments around the world are investing huge amounts of money in drugs. To address one of the greatest medical challenges in recent human memory-COVID-19, pharmaceutical companies around the world have fired up. Although there is still a long way to figure out the pathogenesis of COVID-19, we know that inflammation is a key factor. Therefore, TNFR1 inhibitors may function as promising candidates for the treatment of COVID-19 patients.
References
[1] Ding, Husheng, et al. "CDK2-mediated upregulation of TNFa as a mechanism of selective cytotoxicity in acute leukemia." Cancer Research. 2021.
[2] Speeckaert, Marijn M., et al. "Tumor necrosis factor receptors: biology and therapeutic potential in kidney diseases." American journal of nephrology 36.3 (2012): 261-270.
[3] Gray, Patrick W., et al. "Cloning of human tumor necrosis factor (TNF) receptor cDNA and expression of recombinant soluble TNF-binding protein." Proceedings of the National Academy of Sciences 87.19 (1990): 7380-7384.
[4] Negm, Ola Hamdy El-Shahat. "Investigations of Signalling Pathways Activation by Mutant Tumour Necrosis Factor Receptors." (2011).
[5] Sedger, Lisa M., and Michael F. McDermott. "TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants–past, present and future." Cytokine & growth factor reviews 25.4 (2014): 453-472.
[6] Fischer, Roman, Roland E. Kontermann, and Olaf Maier. "Targeting sTNF/TNFR1 signaling as a new therapeutic strategy." Antibodies 4.1 (2015): 48-70.
[7] Deng, Guo-Min, et al. "Lupus serum IgG induces skin inflammation through the TNFR1 signaling pathway." The Journal of Immunology 184.12 (2010): 7154-7161.
[8] Jones, E. Y., D. I. Stuart, and NPC WALKER. "The structure of tumour necrosis factor-implications for biological function." Journal of Cell Science 1990.Supplement 13 (1990): 11-18.
[9] Li, Kang, et al. "The involvement of TNF-α and TNF-β as proinflammatory cytokines in lymphocyte-mediated adaptive immunity of Nile tilapia by initiating apoptosis." Developmental & Comparative Immunology 115 (2020): 103884.
[10] Wang, Xia, and Yong Lin. "Tumor necrosis factor and cancer, buddies or foes? 1." Acta Pharmacologica Sinica 29.11 (2008): 1275-1288.
[11] Shuh, Maureen, et al. "Tumor necrosis factor-α: life and death of hepatocytes during liver ischemia/reperfusion injury." Ochsner Journal 13.1 (2013): 119-130.
[12] Naudé, Petrus JW, et al. "Tumor necrosis factor receptor cross‐talk." The FEBS journal 278.6 (2011): 888-898.
[13] Gane, Jennie M., Robert A. Stockley, and Elizabeth Sapey. "TNF-α autocrine feedback loops in human monocytes: the pro-and anti-inflammatory roles of the TNF-α receptors support the concept of selective TNFR1 blockade in vivo." Journal of immunology research 2016 (2016).
[14] Van Hauwermeiren, Filip, Roosmarijn E. Vandenbroucke, and Claude Libert. "Treatment of TNF mediated diseases by selective inhibition of soluble TNF or TNFR1." Cytokine & growth factor reviews 22.5-6 (2011): 311-319.
[15] MacEwan, David J. "TNF ligands and receptors-a matter of life and death." British journal of pharmacology 135.4 (2002): 855-875.
[16] Miki, Kiyoshi, and Edward M. Eddy. "Tumor necrosis factor receptor 1 is an ATPase regulated by silencer of death domain."
Molecular and cellular biology 22.8 (2002): 2536-2543.
[17] Li, Hongxiu, and Xin Lin. "Positive and negative signaling components involved in TNFα-induced NF-κB activation." Cytokine 41.1 (2008): 1-8.
[18] Yang, Zijun, et al. "Phosphorylated form of pyruvate dehydrogenase α1 mediates tumor necrosis factor α‑induced glioma cell migration." Oncology Letters 21.3: 1-1.
[19] Teng, Chih-Chuan, et al. "Novel regulator role of CIL-102 in the epigenetic modification of TNFR1/TRAIL to induce cell apoptosis in human gastric cancer." Food and Chemical Toxicology 147 (2020): 111856.
[20] Yun, Hyung-Mun, et al. "IL-32α suppresses colorectal cancer development via TNFR1-mediated death signaling." Oncotarget 6.11 (2015): 9061.
[21] Hwang, Hee Sang, et al. "Involvement of the TNF-α Pathway in TKI Resistance and Suggestion of TNFR1 as a Predictive Biomarker for TKI Responsiveness in Clear Cell Renal Cell Carcinoma." Journal of Korean medical science 35.5 (2020).
[22] Bluemel, Sena, et al. "Tumor necrosis factor alpha receptor 1 deficiency in hepatocytes does not protect from non-alcoholic steatohepatitis, but attenuates insulin resistance in mice." World Journal of Gastroenterology 26.33 (2020): 4933.
CUSABIO team. TNFR1: A Multifaceted Molecule in Cancer, Another Promising Therapeutic Target for COVID-19. https://www.cusabio.com/c-21018.html

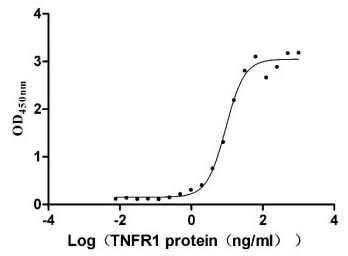
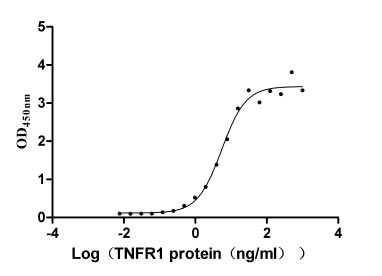



Comments
Leave a Comment