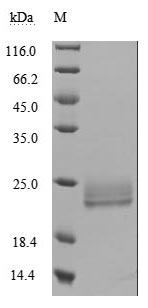
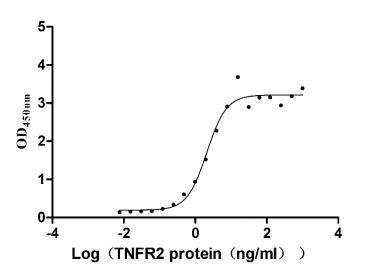
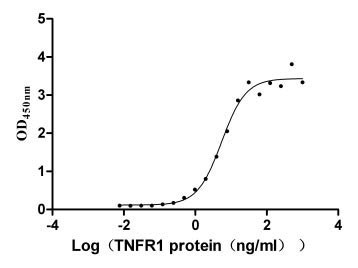
The recombinant human LTA protein is expressed in mammalian cells using a plasmid encoding the 35-205 amino acid region of the human LTA. This LTA protein is co-expressed with the N-terminal 6xHis-tag. The LTA protein achieves a purity of over 95%, as measured by SDS-PAGE, and contains less than 1.0 EU/μg endotoxin based on LAL assay results. ELISA confirms its activity through specific binding to human TNFRSF1B (CSB-MP023978HU2) and human TNFR1 (CSB-MP023977HU1), with the EC50 of 1.632-2.699 ng/ml and 4.409-6.797 ng/ml, respectively.
Human lymphotoxin-alpha (LTA) is a cytokine that plays a critical role in the immune system, particularly in the regulation of immune responses and the maintenance of lymphoid structures. It is primarily produced by activated T and B lymphocytes. LTA is known to form a heterotrimeric complex with LTβ, which is essential for various biological functions, including the organization of lymphoid tissues and the modulation of immune responses [1][2].
LTA is crucial for the development and maintenance of secondary lymphoid organs (SLOs), such as lymph nodes and spleen, by promoting the formation of specialized structures within these organs that facilitate immune cell interactions [1][2]. It has been shown that LTA signaling is vital for the organization of ectopic germinal centers, which are sites of B cell activation and proliferation, particularly in chronic inflammatory conditions [3]. Moreover, LTA is involved in the regulation of T cell responses, enhancing their activation and survival, which is particularly relevant in the context of cancer immunotherapy [4][5].
LTA is involved in the granulomatous response to infections such as tuberculosis, where it helps to organize the immune response necessary for controlling the infection [6]. Furthermore, LTA has been linked to the development of liver inflammation and hepatocellular carcinoma, indicating its potential role in tumorigenesis [7].
References:
[1] D. Liepinsh, S. Grivennikov, K. Klarmann, M. Lagarkova, M. Drutskaya, S. Lockett, et al. Novel lymphotoxin alpha (ltα) knockout mice with unperturbed tumor necrosis factor expression: reassessing ltα biological functions, Molecular and Cellular Biology, vol. 26, no. 11, p. 4214-4225, 2006. https://doi.org/10.1128/mcb.01751-05
[2] J. Šedý, V. Bekiaris, & C. Ware. Tumor necrosis factor superfamily in innate immunity and inflammation, Cold Spring Harbor Perspectives in Biology, vol. 7, no. 4, p. a016279, 2014. https://doi.org/10.1101/cshperspect.a016279
[3] Y. Gao. Rheumatoid arthritis: pathogenesis and therapeutic advances, Medcomm, vol. 5, no. 3, 2024. https://doi.org/10.1002/mco2.509
[4] S. Yang, K. Hayer, H. Fazelinia, L. Spruce, M. Asnani, A. Naqvi, et al. Fbxw7β isoform drives transcriptional activation of the proinflammatory tnf cluster in human pro-b cells, Blood Advances, vol. 7, no. 7, p. 1077-1091, 2023. https://doi.org/10.1182/bloodadvances.2022007910
[5] A. Kamali. Immune checkpoints and cancer immunotherapies: insights into newly potential receptors and ligands, Therapeutic Advances in Vaccines and Immunotherapy, vol. 11, 2023. https://doi.org/10.1177/25151355231192043
[6] J. FITNESS, P. Fine, A. Crampin, D. Warndorff, S. Floyd, S. MALEMA, et al. Large-scale candidate gene study of tuberculosis susceptibility in the karonga district of northern malawi, American Journal of Tropical Medicine and Hygiene, vol. 71, no. 3, p. 341-349, 2004. https://doi.org/10.4269/ajtmh.2004.71.341
[7] S. Cairo and M. Buendia. How transient becomes stable: an epigenetic switch linking liver inflammation and tumorigenesis, Journal of Hepatology, vol. 57, no. 4, p. 910-912, 2012. https://doi.org/10.1016/j.jhep.2012.05.017