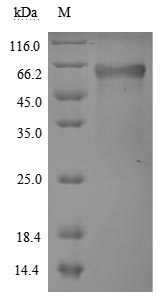
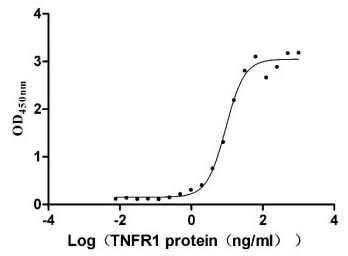
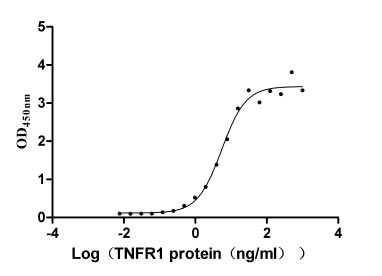
The recombinant human TNFRSF1A protein is expressed in mammalian cells through a plasmid construct containing the gene segment encoding the 22-211aa of the human TNFRSF1A. The TNFRSF1A protein carries a C-terminal hFc-tag. Its purity is greater than 90% as confirmed by SDS-PAGE. Its endotoxin levels, measured using the LAL method, are less than 1.0 EU/μg. ELISA verifies the biological activity of the TNFRSF1A protein through specific binding to the TNF-α (CSB-YP023955HU) and LTA(CSB-MP013218HU), yielding the EC50 of 7.799-10.90 ng/mL and 4.409-6.797 ng/mL, respectively.
Human TNFRSF1A, also known as TNFR1, is a transmembrane glycoprotein expressed in nearly all human cells except erythrocytes, with notable expression in immune cells, endothelial cells, and cells of the hematopoietic lineage [1]. TNFRSF1A mediates various biological processes, including inflammation, apoptosis, and immune responses, by binding to TNF-α and TNF-β [2][3].
Upon activation by TNF-α, TNFRSF1A initiates a cascade of intracellular signaling pathways, notably the NF-κB pathway, which is crucial for cell survival and proliferation [4]. TNFRSF1A also modulates inflammatory responses as it is involved in several autoimmune and inflammatory diseases such as multiple sclerosis and ankylosing spondylitis [2][5]. Genetic variants in TNFRSF1A have been associated with susceptibility to these conditions [6][7]. Mutations in the TNFRSF1A gene have been identified in patients with tumor necrosis factor receptor-associated periodic syndrome (TRAPS), leading to dysfunctional receptor signaling and impaired apoptosis in immune cells [6][8][9].
In cancer biology, TNFRSF1A has been recognized as a potential biomarker for various malignancies, including gliomas and osteosarcoma [3][10]. Its expression levels can influence tumor behavior and patient prognosis, making it a target for therapeutic interventions [3][10]. Furthermore, the receptor's interaction with microRNAs, such as miR-29a, highlights its regulatory role in vascular smooth muscle cells and its potential implications in atherosclerosis and post-stroke depression [11][12].
References:
[1] S. Zhou, W. Zhao, J. Liu, Y. Hong-yuan, J. Yang, Q. Wang, et al. Bioinformatics analysis identifies tnfrsf1a as a biomarker of liver injury in sepsis tnfrsf1a is a biomarker for septic liver injury, Genetics Research, vol. 2022, p. 1-10, 2022. https://doi.org/10.1155/2022/1493744
[2] V. Leppä, I. Surakka, P. Tienari, I. Elovaara, A. Compston, S. Sawcer, et al. The genetic association of variants in cd6, tnfrsf1a and irf8 to multiple sclerosis: a multicenter case-control study, Plos One, vol. 6, no. 4, p. e18813, 2011. https://doi.org/10.1371/journal.pone.0018813
[3] B. Yang, Y. Pan, Y. Ma, & S. Chu. Integrated transcriptome analyses and experimental verifications of mesenchymal-associated tnfrsf1a as a diagnostic and prognostic biomarker in gliomas, Frontiers in Oncology, vol. 10, 2020. https://doi.org/10.3389/fonc.2020.00250
[4] S. Egusquiaguirre, J. Yeh, S. Walker, S. Liu, & D. Frank. The stat3 target gene tnfrsf1a modulates the nf-κb pathway in breast cancer cells, Neoplasia, vol. 20, no. 5, p. 489-498, 2018. https://doi.org/10.1016/j.neo.2018.03.004
[5] S. Davidson, Y. Liu, P. Danoy, X. Wu, G. Thomas, L. Jiang, et al. Association of stat3 and tnfrsf1a with ankylosing spondylitis in han chinese, Annals of the Rheumatic Diseases, vol. 70, no. 2, p. 289-292, 2010. https://doi.org/10.1136/ard.2010.133322
[6] P. Jager, X. Jia, J. Wang, P. Bakker, L. Ottoboni, N. Aggarwal, et al. Meta-analysis of genome scans and replication identify cd6, irf8 and tnfrsf1a as new multiple sclerosis susceptibility loci, Nature Genetics, vol. 41, no. 7, p. 776-782, 2009. https://doi.org/10.1038/ng.401
[7] J. Sode, S. Bank, U. Vogel, P. Andersen, S. Sørensen, A. Bojesen, et al. Genetically determined high activities of the tnf-alpha, il23/il17, and nfkb pathways were associated with increased risk of ankylosing spondylitis, BMC Medical Genetics, vol. 19, no. 1, 2018. https://doi.org/10.1186/s12881-018-0680-z
[8] E. Aganna, I. Aksentijevich, G. Hitman, D. Kastner, A. Hoepelman, F. Posma, et al. Tumor necrosis factor receptor-associated periodic syndrome (traps) in a dutch family: evidence for a tnfrsf1a mutation with reduced penetrance, European Journal of Human Genetics, vol. 9, no. 1, p. 63-66, 2001. https://doi.org/10.1038/sj.ejhg.5200573
[9] A. D’Osualdo, F. Ferlito, I. Prigione, L. Obici, A. Meini, F. Zulian, et al. Neutrophils from patients with tnfrsf1a mutations display resistance to tumor necrosis factor–induced apoptosis: pathogenetic and clinical implications, Arthritis & Rheumatism, vol. 54, no. 3, p. 998-1008, 2006. https://doi.org/10.1002/art.21657
[10] Y. Zhang. Identification of tnfrsf1a as a potential biomarker for osteosarcoma, Cancer Biomarkers, vol. 39, no. 4, p. 299-312, 2024. https://doi.org/10.3233/cbm-230086
[11] L. You, H. Chen, L. Xu, & X. Li. Overexpression of mir-29a-3p suppresses proliferation, migration, and invasion of vascular smooth muscle cells in atherosclerosis via targeting tnfrsf1a, Biomed Research International, vol. 2020, p. 1-15, 2020. https://doi.org/10.1155/2020/9627974
[12] M. Wang, J. Guo, D. Liu, & J. Wang. Cerebellar fastigial nucleus stimulation in a chronic unpredictable mild stress rat model reduces post-stroke depression by suppressing brain inflammation via the microrna-29c/tnfrsf1a signaling pathway, Medical Science Monitor, vol. 25, p. 5594-5605, 2019. https://doi.org/10.12659/msm.911835