GPRC5D (G Protein-Coupled Receptor Class C Group 5 Member D) has emerged as a promising therapeutic target, garnering significant attention in the field of oncology and immunotherapy. Particularly in the treatment of multiple myeloma (MM), GPRC5D has become a focal point of research due to its unique biological properties and potential therapeutic advantages. In 2023, a major milestone was reached when Amgen, a global pharmaceutical leader, entered into a substantial collaboration with a biotechnology firm, committing hundreds of millions of dollars to jointly develop a GPRC5D-targeted CAR-T cell therapy. This strategic partnership underscores the immense potential of the GPRC5D target and signals an intensifying global competition in its development.
Moreover, recent preclinical studies have demonstrated high expression of GPRC5D in various hematologic malignancies, positioning it as a potential next-generation breakthrough target following CD19, CD38, and BCMA. As more clinical trials advance and data emerge, GPRC5D holds the promise of offering new therapeutic options for patients with multiple myeloma and potentially reshaping the treatment landscape of hematologic cancers.
1. What is GPRC5D?
GPRC5D (G protein-coupled receptor, class C, group 5, member D) is one of the members of the G protein-coupled receptor (GPCRs) family. In recent years, GPCRs are emerging as important therapeutic targets, which shed new light on drug discovery. To date, nearly 2000 species of G protein-coupled receptors have been reported, which are the most widely distributed and important membrane protein receptors in the human body. GPRC5D, as a novel GPCR therapeutic target, its endogenous ligand is still unknown, which namely belongs to an orphan receptor. GPRC5D is widely expressed in malignant bone marrow plasma cells, hair follicles, and lungs, whereas in normal tissues, GPRC5D expression is very limited, with no or only low expression. Given the fact that GPRC5D is specifically highly expressed in multiple myeloma (MM) [1], it has been considered as a new drug target in MM therapy. Presently, GPRC5D is attracting increased attention from many researchers and pharmaceutical companies. Currently, several drugs targeting GPRC5D or GPRC5D-based combinations are in clinical phase. These newly GPRC5D-targeted drugs are designed to treat multiple myeloma (MM). The researchers anticipate that GPRC5D could be the next ideal candidate for the MM treatment.
2. What is Multiple Myeloma (MM)?
What is multiple myeloma? Multiple myeloma (MM) is a type of plasma cell neoplasms. Plasma cells are found in the bone marrow, which accounts for the risk of developing an autoimmune disease. When malignant proliferation of plasma cells occurs in the bone marrow, it will trigger multiple myeloma. As a serious hematologic disease, multiple myeloma can destroy the bones and the immune system with a wide variety of clinical manifestations, such as bone pain, osteoporosis, renal lesions, lung inflammation, hypercalcemia, etc [2]. Apparently, there is a notable unmet medical needs for multiple myeloma, in particular, MM patients with BCMA-negative relapse without better treatment strategy yet. With emerging therapeutic targets, researchers have turned their attention to a novel target of GPCRs family, GPRC5D.
3. GPRC5D Treatment Strategy in Multiple Myeloma (MM)
The therapy of multiple myeloma (MM) has seen remarkable progress over the past years. There has been a major shift in the choice of agents to treat multiple myeloma, including proteasome inhibitors, immunomodulators, monoclonal antibodies and chimeric antigen receptor cell (CAR-T) therapies. However, this positive news is tempered by the realization that these measures are not curative and patients eventually relapse and/or become resistant to the drug's effects. As previously mentioned, the drug efficacy is not promising in relapsed cases with low or negative BCMA expression. Intriguingly, it was found that GPRC5D is independently expressed with BCMA, which means that GPRC5D can be targeted either singly or in combination with dual-targeted therapeutics [3], which offer a new therapeutic strategy for MM patients. At present, therapeutic strategies for GPRC5D in multiple myeloma (MM) are focused on GPRC5D CAR-T cell therapy and GPRC5D based bispecific antibody therapy.
3.1 GPRC5D CAR-T Cell Therapy
The MM clinical treatment study of GPRC5D CAR-T first came from Memorial Sloan Kettering Cancer Center (MSKCC) and Eureka Therapeutics Inc. In 2019, scientists at MSKCC and Eureka published the results of GPRC5D CAR-T study, suggesting that GPRC5D is expressed on malignant bone marrow plasma cells, with limited expression in normal tissue of the hair follicle region. Their findings further identified that GPRC5D-targeted CAR-T therapy can curb BCMA antigen escape-mediated relapse in a murine model, confirming the independent expression of GPRC5D and BCMA [3, 5].
The study is a sign that GPRC5D CAR-T holds the great potential for multiple myeloma treatment, especially for patients who have relapsed after other treatments. In 2021, global pharmaceutical leader Sanofi puts $ 1B-plus on the table for Eureka’s multiple myeloma treatment. Last month, the first domestic GPRC5D CAR-T also launched a Phase I clinical trial for multiple myeloma or leukemia.
3.2 GPRC5D Based Bispecific Antibody Therapy
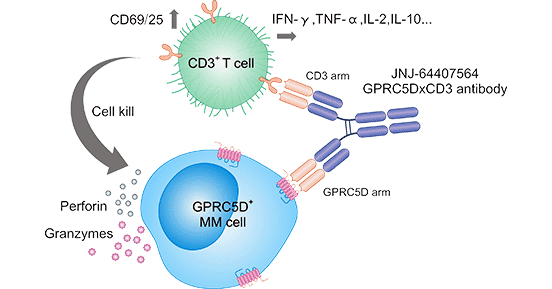
The clinical treatment of MM with GPRC5D bispecific antibodies has been studied primarily by Johnson & Johnson and Eureka Therapeutics Inc. In 2021, Johnson & Johnson presented early clinical data of GPRC5DxCD3 bispecific antibody Talquetamab (JNJ-64407564) at the ASCO meeting, which demonstrated good efficacy in the treatment of MM. This humanized GPRC5DxCD3 bispecific antibody targets both CD3 and GPRC5D. GPRC5D is highly expressed on multiple myeloma cells. CD3 is a protein complex and T cell co-receptor that is involved in activating both the cytotoxic T cell. GPRC5DxCD3 bispecific antibody, by binding to the CD3 receptor on the surface of T cells, recruits T cells to tumor cells, which in turn attack MM tumor cells with high GPRC5D expression. In other words, GPRC5DxCD3 bispecific antibodies recruit CD3-expressing T cells to GPRC5D-expressing MM cells, resulting in the activation of the T-cell receptor (TCR) signaling pathway, thereby inhibiting MM cell growth [4].
Figure 1. The mechanism of GPRC5DxCD3 regulates MM cells
4. Recent Advances in GPRC5D-Targeted Treatment
Although GPRC5D was discovered in the beginning, from the published clinical data, GPRC5D exhibited good efficacy and considerable potential in multiple myeloma (MM) treatment. Currently, there are four GPRC5D targeted drugs under research, mainly for multiple myeloma treatment: MCARH109 GPRC5D CAR T-cell therapy is in phase I clinical trial, from Sanofi, Eureka Therapeutics Inc. and MSKCC; Nanjing Reindeer Medical Technology Co., Ltd., recently registered GPRC5D CAR-T study in ClinicalTrials.gov website, which is in phase I clinical trial; Talquetamab, a GPRC5DxCD3 bispecific antibody developed by Johnson & Johnson, clinical phase II, of which the phase I data indicated that the overall response rate (ORR) of Talquetamab up to 69%; BCMAxGPRC5D dual-targeted CAR-T therapy is being explored by Eureka Therapeutics Inc., currently under in the preclinical stage.
Overall, GPRC5D has proven to be a very fruitful target for MM, the clinical pipelines are in early stage though. Importantly, as newly discovered target, GPRC5D is typically highly expressed on MM cells. Several clinical trials confirm that GPRC5D shown to be efficacious in MM patients. Notably, studies indicated that GPRC5D CAR T would be a better strategy to prevent BCMA escape-driven relapse, which is expected to improve the long-term clinical benefit for MM patients. Therefore, GPRC5D targeting will be a promising approach for MM treatment, especially for those in relapsed/refractory stage.
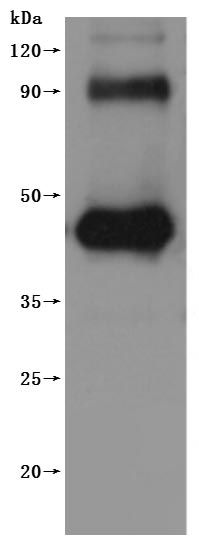
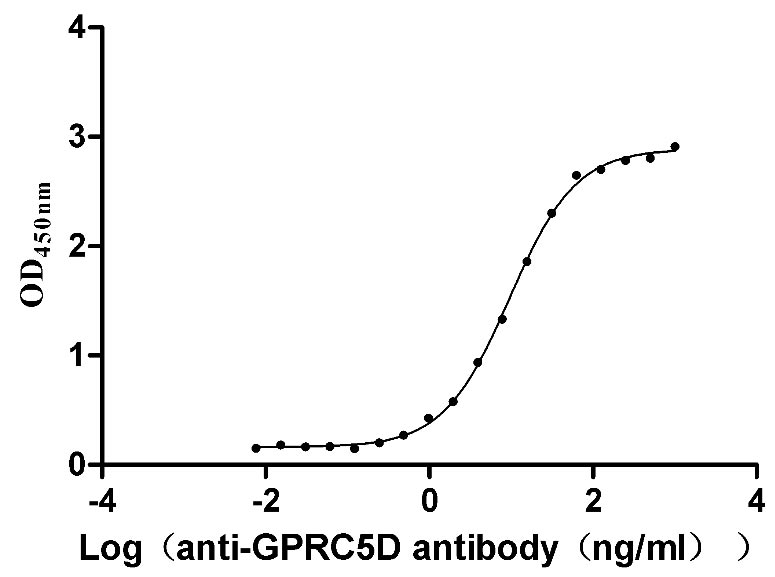
To fully serve the pharmaceutical companies in GPRC5D based drug research for multiple myeloma (MM) treatment. CUSABIO has launched the GPRC5D-VLPs active protein product (Code: CSB-MP882153HU) to help you explore the mechanism of GPRC5D or its potential clinical value.
References
[1] Pillarisetti, Kodandaram, et al. "A T-cell-redirecting bispecific G-protein-coupled receptor class 5 member D x CD3 antibody to treat multiple myeloma." Blood 135.15 (2020): 1232-1243.
[2] Cohen, Yossi, et al. "GPRC5D is a promising marker for monitoring the tumor load and to target multiple myeloma cells." Hematology 18.6 (2013): 348-351.
[3] Smith, Eric L., et al. "GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. "Science translational medicine 11.485 (2019): eaau7746.
[4] Kodama, Tatsushi, et al. "Anti-GPRC5D/CD3 bispecific T-cell-redirecting antibody for the treatment of multiple myeloma." Molecular cancer therapeutics 18.9 (2019): 1555-1564.
[5] De Larrea, Carlos Fernández, et al. "Defining an optimal dual-targeted CAR T-cell therapy approach simultaneously targeting BCMA and GPRC5D to prevent BCMA escape-driven relapse in multiple myeloma." blood cancer discovery 1.2 (2020): 146.
CUSABIO team. GPRC5D: Newly Efficient GPRC5D CAR-T or Bispecific Antibody Therapy to Fight Multiple Myeloma (MM). https://www.cusabio.com/c-21063.html




Comments
Leave a Comment