"Porcelain doll", "sticky baby" ... behind these seemingly beautiful words is the abyss of pain. It is reported that more than 350 million people in the world are suffering from rare diseases. Statistics show that the prevalence rate of rare diseases in the population is about 3.5%-5.9%. Although the prevalence rate of about 84% of diseases is less than one in one million, the total number of patients with rare diseases in China has reached about 20 million, and the annual growth rate of more than 200 thousand patients. Compared with other countries and regions, many rare diseases have become "uncommon" in China. There are a variety of rare diseases, and more than 10,000 diseases are known currently, most of which are chronic diseases that are difficult to prevent or treat, with high cost burden, and often lead to premature death of patients. In recent years, many countries have promulgated a series of rare disease-related legislation and decisions, aiming to encourage the introduction, research and development and production of rare disease drugs, and speed up the registration, evaluation and approval of rare disease drugs.
The development of drugs for rare diseases seems to have spread all over the world. So, what is a rare disease? How to treat rare diseases? What is its drug market?
1. What is a rare disease?
Rare disease is also called "orphan disease", and the World Health Organization (WHO) defines the disease with an incidence rate of 0.65 ‰–1 ‰ as a rare disease. At present, there are more than 10,000 known rare diseases worldwide [1], accounting for about 10% of known human diseases and involving nearly 400 million patients, which means that one in 17 people will suffer from a rare disease at some stage in their lifetime. The main body of rare diseases is children, and 80% of rare diseases are hereditary diseases. In addition, rare diseases are characterized by complexity, severity and deterioration, which impose heavy physical, psychological and economic burdens on patients. They are one of the major problems that need to be solved in modern medicine.
2. Challenges faced by rare diseases
Unfortunately, rare illnesses have remained low on the public health agenda over the past decades. It is not surprising that, taken alone, the health burden of a single rare disease is too small to be a simple health economy case; But as a group, rare illnesses are almost as common as common respiratory illnesses such as asthma. The lack of expertise in the field of rare diseases means that patients often have to wait years to be diagnosed and are often even misdiagnosed and subjected to unnecessary invasive examinations. Clinicians often come into contact with some patients with fever of unknown cause, and carry out a large number of biochemical tests and imaging examinations, but the results are nothing. The patients are discharged without a definite diagnosis. At the level of the broader health system, the economic cost of rare diseases is undoubtedly huge. It is estimated that patients with rare illnesses during diagnosis have spent more than 3.4bn on the NHS in the past decade, with 10 percent of the NHS budget in 2016 being spent on rare illness diagnoses. In addition, patients with rare diseases each spend about 13000 pounds in 10 years, which is almost double the cost related to other more common diseases [2].
3. Therapeutic research of rare diseases
There are more than 10,000 known rare diseases, and only about 1% of them have effective therapeutic drugs. This is because rare diseases often have unique pathogenesis and complex research and development process. Therefore, it is necessary to find new drugs with new mechanisms of action (MOA). At the same time, the MOA research on orphan drugs often leads to the discovery of new drug targets, which greatly promotes the research and development process of the first-in-class (FIC) drug.
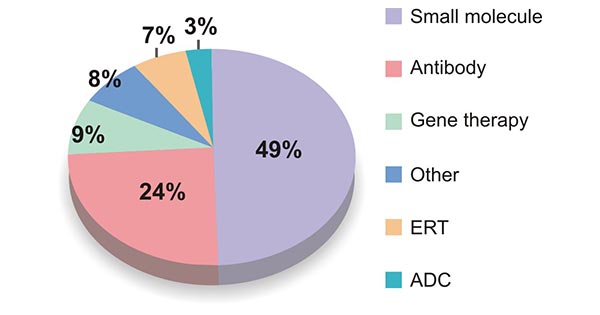
There are a variety of rare diseases. At present, common treatments for rare diseases include small molecule drugs, monoclonal antibodies, enzyme replacement therapy (ERT), oligonucleotide therapy, stem cell transplantation and innovative therapies based on the next generation suitable for genetic defects. The figure below shows the statistics of the types of drugs used to treat rare diseases for the period 2011 to 2020.
Figure 1 Percentage of drug types of FIC orphan drugs that approved in 2011-2020 [3]
● Small molecule drug
Small molecules are usually the most recognized drug platforms for diseases, and they are very attractive as therapeutic agents due to the variety of drug delivery routes, good stability, controlled dose and low cost. However, progress in small molecule drugs is slow, and new screening techniques and synthetic chemistry, computational screening, and structural biological rate are accelerating that discovery and design of new bioactive molecules.
● Antibody drug
In 1986, the FDA approved Orthoclone OKT3, the first therapeutic monoclonal antibody drug, which kicked off the development of monoclonal antibody drugs. Today, more than 30 years have passed since biopharmaceuticals. With the improvement of humanization of monoclonal antibodies, the efficacy of monoclonal antibodies has been better played. So far, monoclonal antibodies have been approved for the treatment of a variety of diseases, including rare diseases. For cancer and immune diseases, antibodies act by regulating signaling pathways that recruit cells or proteins to specific sites, deliver cytotoxins, or neutralize or modulate circulating factors.
The main advantage of antibody therapy is its high specificity, which reduces the risk of off-target toxicity commonly seen in small molecule preparations and is essential for the treatment of rare diseases. Currently, several monoclonal antibodies have been approved for the treatment of rare diseases.
● Enzyme replacement therapy (ERT)
Enzyme replacement therapy is the periodic injection of a natural or recombinant enzyme into a patient to alleviate the deficiency or dysfunction of endogenous enzymes. Is mainly use for treating partial lysosomal storage disease. Successful ERT applications include Fabry's disease, Gaucher's disease and Hunter's syndrome.
● Gene therapy
Gene therapy is currently the most cutting-edge treatment technology for rare diseases for which the causative gene mutation can be identified. Gene Therapy refers to the replacement of a mutated gene by introducing a normal gene into a patient's body cell, or producing functional proteins to restore the normal function of cells, thereby achieving the purpose of treating diseases.
4. Market of rare disease drugs
Drugs to treat rare illnesses are also called "orphan drugs". In recent years, under the encouragement of a series of preferential policies, the rare disease market has continued to heat up. According to Frost&Sullivan, the global market for rare disease drugs will increase from US$ 135.1 billion in 2020 to US$ 383.3 billion in 2030, with an annual compound growth rate of 11%. This means that there is a huge market potential in the rare disease field. As a result, more and more multinational pharmaceutical companies have entered the rare disease market and accelerated the acquisition and expansion of rare disease drug research and development pipeline. According to the Pharmaceutical Research and Manufacturers of America, there were approximately 791 potential rare disease treatment drugs under clinical trial in 2021. Of the clinical medicines for all rare diseases, 168 are used for rare cancers, 120 are used for rare hematological tumors, 192 are used for hereditary diseases, 56 are used for nervous system diseases, 54 are used for hematological diseases, 51 are used for autoimmune diseases and 36 are used for infectious diseases.
Among the TOP10 pharmaceutical companies with global revenue announced in 2021, almost all of them, including Johnson & Johnson, Pfizer and Roche, have actively deployed in the field of rare diseases. In addition, Takeda, which has been paying close attention to drugs for rare diseases, has designated about 50% of the new compound research and development projects in nearly 40 clinical stages worldwide as orphan drugs.
5. Rare diseases-related antibody drugs
The following is a compilation of rare disease antibody drugs that have been approved and marketed worldwide in recent years.
● Burosumab
Burosumab, the only drug approved to address the underlying cause of X-linked hypophosphatemic rickets (XLH), was jointly developed by Kyowa Hakko Kirin (Japan) and Ultragenyx Pharmaceutical. XLH is a group of rare bone mineralization disorders characterized by hypophosphatemia caused by increased renal phosphorus excretion due to genetic or acquired causes, which will cause a lifelong burden on patients. Burosumab is a recombinant fully human IgG1 monoclonal antibody targeting fibroblast growth factor 23 (FGF23) antigen. Burosumab can target and inhibit the activity of FGF23, inhibit its downstream signaling pathways, increase renal reabsorption of phosphorus and serum active vitamin D levels, and promote intestinal absorption of phosphate and calcium. Increased serum phosphorus levels ultimately improve bone mineralization and reduce bone disease.
● Satralizumab
In June 2020, Roche announced that the European Commission (EC) had approved the use of Satralizumab for the treatment of neuromyelitis optica spectrum disorders (NMOSD) either as monotherapy or in combination with immunosuppressive therapy. NMOSD is a rare, highly recurrent and disabling autoimmune disease. Satralizumab is a humanized IgG2 monoclonal antibody developed by SMARTTM recycling technology. Satralizumab targets the IL-6 receptor and can inhibit the production of NMOSD specific antibody AQP4-IgG and inflammatory response in and out of the central nervous system. On May 8, 2021, Satralizumab was officially approved for marketing in China, becoming the first NMOSD treatment drug in China.
● Mepolizamab
Mepolizamab, originally developed by GlaxoSmithKline (GSK), is the world's first approved biologic therapy targeting IL-5. On 17 November 2021, National Medical Products Administration, China (NMPA) first granted approval for the marketing of mepolizumab in China for the indication of eosinophilic granulomatous polyangiitis (EGPA) in adult patients. EGPA is a rare autoimmune disease. EGPA is heterogeneous in presentation and is caused by vasculitis and/or eosinophilic tissue inflammation that can lead to multiple organ damage, including lung, skin, cardiovascular and nervous system, posing a serious life-threatening condition. Mepolizamab can selectively recognize and bind IL-5, block its binding to eosinophil surface receptors, and reduce the growth of eosinophils, thereby reducing eosinophil-mediated inflammation and tissue damage and maintaining a healthy state.
● Daratumumab
Daratumumab is the world's first and only approved innovative solution for the treatment of patients with systemic light-chain amyloidosis. Approved by NMPA in October 2021 for the treatment of a newly diagnosed patient with primary light-chain amyloidosis with bortezomib, cyclophosphamide, and dexamethasone. Primary light-chain amyloidosis is a rare and fatal hematological disease in which abnormal amyloid light chains are produced and constantly accumulated in vital organs by plasma cells in the patient's bone marrow, eventually leading to organ failure or even death in the patient. Daratumumab is the first fully human monoclonal antibody against CD38 approved in the world and China. It has a broad spectrum of killing activity and can bind to the transmembrane extracellular enzyme CD38, which is highly expressed on the surface of multiple myeloma cells, and induce rapid death of tumor cells through a variety of mechanisms.
● Eculizumab
Eculizumab, marketed as Soliris, is a recombinant humanized monoclonal antibody that inhibits terminal complement C5 and is currently approved clinically for the treatment of rare hematologic disorders, including paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS). PNH is a hemolytic disease characterized by chronic intravascular hemolysis, hematopoietic failure and recurrent thrombosis. aHUS can cause progressive damage to vital organs, mainly the kidneys, through destruction of the vessel wall and blood clots. Eculizumab has a high affinity for C5, capable of C5 binding and hindering C5 cleavage by C5 convertase. Because complement activation after C5 convertase is a common pathway of all complement activation pathways, eculizumab can inhibit the complement activation process and the formation of its end products through three pathways, and prevent cell lysis.
● Ofatumumab
In December 2021, NMPA approved the use of Ofatumumab injection for the treatment of relapsed multiple sclerosis (RMS) in adults, including clinical isolated syndrome, relapsed remission multiple sclerosis, and active secondary progressive multiple sclerosis. Multiple sclerosis (MS) is a severe, lifelong, progressive, disabling, and rare central nervous system disease with major manifestations of muscle weakness, fatigue, pain, cognitive impairment, and visual problems, and has become the main cause of non-traumatic disability. Ofatumumab injection is a fully human immunoglobulin G1 monoclonal antibody against human CD20, which can target CD20 molecule and achieve the therapeutic effect on MS by inducing B cell lysis.
6. Prospect
According to the report entitled "Drug Payment for Rare Diseases in China under Common Prosperity", as of July 2022, a total of 180 clinical studies of new drugs targeting the diseases in the first batch of rare disease lists were conducted in China, covering a total of 36 rare diseases. The research scope covered both the upgrade of existing disease treatment methods and the challenge to the "no drug around the world" treatment dilemma.
It is not difficult to see that the research on rare diseases has entered an encouraging era. Many drugs under development carry the good expectations of patients with related rare diseases worldwide for effective drug treatment. It is believed that in the near future, rare diseases will become the key areas for pharmaceutical enterprises to seek innovative breakthroughs and rapid rise.
References
[1] https://project8p.org/rare-disease-facts/
[2] https://imperialcollegehealthpartners.com/new-report-reveals-undiagnosed-rare-disease-patients-cost-nhs-excess-3-4-billion/
[3] Gu J Y, Wu Q Y, Zhang Q Y , et al. A decade of approved first-in-class small molecule orphan drugs: Achievements, challenges and perspectives[J]. European Journal of Medicinal Chemistry, 2022, 243
CUSABIO team. Categories over 10000! --Rare diseases that are no longer . https://www.cusabio.com/c-21102.html



Comments
Leave a Comment