TPBG/5T4 has been so appealing recently to pharmaceutical companies as it yields encouraging results in tumor therapies. ClinicalTrial noted that there are over 10 TPBG-targeted drugs in clinical development. Notably, Oxford BioMedica's TroVax, an MVA-based vaccine targeting TPBG has been evaluated in phase II and/or phase III clinical trials in various tumors, demonstrating that patients given TroVax show "significant anti-5T4 immune responses". In addition, TPBG-targeted antibody-drug conjugate (ADC) drugs have also been applied for tumor treatments. For example, Asana BioSciences' ASN004, a novel TPBG-targeted Dolaflexin™ ADC, the results from ASN004 preclinical works indicated that ASN004 achieves complete regressions and tumor-free survivors in solid tumor models.
Basically, TPBG plays vital roles during embryonic development, but it is also an extensively investigated oncofetal antigen. Given that, it drives the clinical development of various TPBG/5T4-targeted therapies including a vaccine, an antibody-targeted superantigen, antibody-drug conjugates (ADC) and chimeric antigen receptor (CAR) T-cell immunotherapies. To this end, TPBG becomes an attractive target for immune intervention in various tumors!
1. What's TPBG?
1.1 TPBG Structure
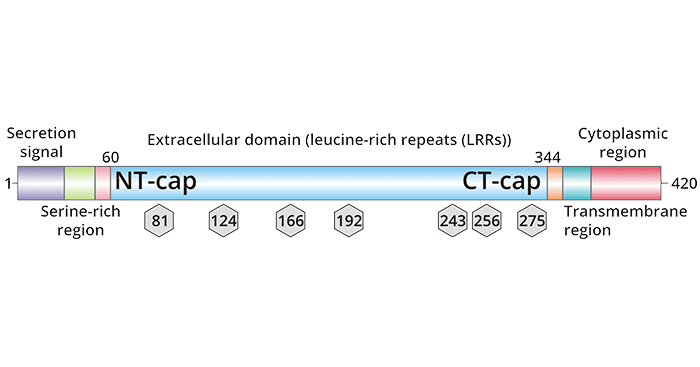
Trophoblast glycoprotein (TPBG, also termed as 5T4 or WAIF1) is a glycoprotein found in embryonic trophoblast cells. TPBG is a 72-kDa transmembrane glycoprotein, comprised of a 310-amino-acid extracellular domain, a 20 amino acid transmembrane region, and a 44-amino-acid cytoplasmic tail, which can influence the cytoskeletal reconstruction and the integrity of cell-cell via its PDZ domain.
The extracellular domain of TPBG contains seven consensus N-glycosylation sites. The extracellular domain also contains six leucine-rich repeat (LRR) sequences that are generally believed to function in protein-protein interactions (Figure 1) [1]. Abundant evidence indicated that TPBG is closely associated with different physiological and pathological processes, such as intercellular junctions, cell morphology and motility, cell adhesion capacity, cell membrane integrity, etc [2].
Figure 1. TPBG Structure [1]
1.2 TPBG Expression
TPBG is relatively widely expressed during embryonic development and is strongly expressed in placental trophoblast cells throughout pregnancy. Besides, studies revealed that embryonic expression of murine TPBG is associated with morphogenetic events at implantation and in developing epithelia. Generally, TPBG is limited in normal adult tissues, but highly expressed in various types of cancer in humans [3, 4].
In normal tissues TPBG is barely present only in certain specialized epithelia, such as basal lamina complex squamous epithelium, glandular and ductal epithelia, and retinal secondary neurons and olfactory bulbs. Currently, TPBG is often referred to as an oncofetal antigen due to its expression in foetal trophoblast. More research is coming out that suggests TPBG is expressed in a number of tumors, like colorectal, gastric and ovarian cancers [3, 4].
1.3 TPBG Function
TPBG not only plays an important role in embryonic cell development, implantation and implantation, but it can guide cell differentiation and embryonic organogenesis during the embryonic stage . TPBG was discovered as researchers were trying to identify shared cell surface molecules, which may function to allow survival of the foetus as a semi-allograft in the mother, or a tumour in its host [3-5].
The shared expression would reflect common functions relevant to growth, invasion, or altered immune surveillance in the host. Intriguingly, successive studies have uncovered that TPBG is highly expressed in a variety of solid tumors while rarely present in normal mature tissues. Therefore, it is considered as a tumor-associated antigen and becomes an up-coming target for anti-cancer drugs design [3-5].
2. How's the Mechanism of Action of TPBG in Tumors?
At present, the molecular mechanisms of TPBG involved in the development of tumorigenesis have not been fully clarified. Existing studies suggest that TPBG has functional attributes that are relevant to tumors including through i) epithelial-mesenchymal transition (EMT) process, ii) regulation of the CXCR4/CXCL12 axis, iii) TPBG-mediated Wnt signaling [5-7].
2.1 TPBG and Epithelial-Mesenchymal Transition (EMT)
The epithelial-mesenchymal transition (EMT) is important for embryonic development and the formation of various tissues or organs. However, EMT dysfunction in normal cells leads to diseases, such as cancer. The most important event of EMT is loss of E-cadherin. E-cadherin ensures cell adhesion and polarity formation by binding to intracytoplasmic β-catenin [5, 6].
The spontaneous differentiation of mouse embryonic stem cells shows features related to an EMT including an E- to N-cadherin switch, upregulation of E-cadherin repressors Snail and Slug, gelatinase activity (MMP2 and MMP9), and increased cellular motility. Studies showing that TPBG and N-cadherin knockout embryonic stem cells suggest remarkably reduced motility during EMT are consistent with a functional role for these proteins [5, 6].
2.2 TPBG and CXCR4/CXCL12 Axis
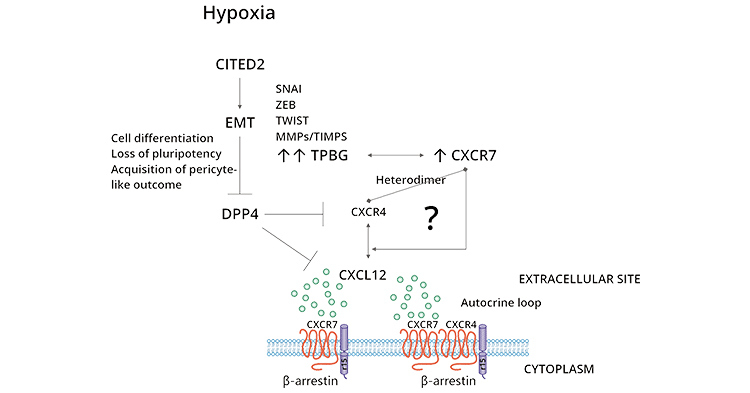
The identification of a link between TPBG and CXCR4/CXCL12 was discovered. It has been found that anti-mTPBG antibodies inhibit the chemotaxis of differentiated murine embryonic stem cells by CXCL12. TPBG facilitates functional CXCR4 expression leading to CXCL12 mediated chemotaxis in mouse embryonic cells. CXCR4/CXCL12 has been shown to be employed in various processes such chemotaxis, invasion, angiogenesis, and proliferation (Click to view a feature on Cytokines) [4, 5, 7].
Using wild type and TPBG knockout murine embryonic fibroblasts (MEFs), it was shown that CXCL12 binding to CXCR4 activates both the ERK and AKT pathways. Importantly, in the absence of TPBG expression, CXCL12 binds CXCR7 which is upregulated, activating Ras-Raf-MAPK and P13K-Akt pathways. Thus, TPBG may promote chemotaxis of tumor cells through the CXCR4/CXCL12 axis, or then further selectively expresses CXCR7 to promote tumor cell proliferation via the CXCR7/CXCL12 axis [4, 5, 7].
Figure 2. TPBG-related signaling pathways [4]
2.3 TPBG and Wnt signaling
Wnt protein signaling is pivotal in the developing embryo and for adult tissue homeostasis, while aberrant signaling is associated with disease including cancer. It has been shown that TPBG expression can inhibit the Wnt/β-catenin canonical pathway. But at the same time, TPBG is able to activate the noncanonical Wnt signaling pathway associated with increased motility [5, 9].
Interference with canonical signaling occurs by binding of TPBG to the Wnt co-receptor LRP6 inhibiting the necessary Wnt induced LRP6 internalization leading to activation of the Wnt-β-catenin pathway. On the flip side, TPBG enhances the β-catenin independent Wnt signaling through promoting a noncanonical function of Dickkopf-1 (DKK1) influencing the actin and microtubular skeleton. It is likely that the integrated TPBG regulation of both the chemokine and Wnt pathways acts to promote cancer spread [5, 9].
3. The Roles of TPBG in Tumors
TPBG has been identified as the oncofetal antigen, which is a shared surface molecule of human trophoblast and many different cancer cells. It was hypothesized that such molecules could have common functions relevant to the survival of the fetus as a semi-allograft in the mother or a tumor in its host including growth, invasion, or altered immunosurveillance. Therefore, the relationship between TPBG and tumors has attracted widespread attention.
3.1 TPBG with Colorectal and Gastric Cancer
In colorectal and gastric cancers, it was shown that TPBG expression was highly correlated with tumor metastasis. And TPBG abnormal expression was mostly found in basal cells in the early stages of the tumor. For example, the TPBG antigen phenotype of 72 colorectal cancers has been compared with the clinical outcome of the patients in order to assess its relationship with prognosis. Forty per cent of tumors were TPBG positive [9].
Besides, there was a significant correlation between TPBG expression in the malignant cells and unfavourable course of disease [9, 17]. The first therapeutic cancer vaccine targeting TPBG (MVA-5T4/Trovax) developed from Oxford BioMedica, UK, was applied in metastatic colorectal cancer (mCRC), which suggested that MVA-5T4 was safe and well tolerated with no serious adverse effects [10].
3.2 TPBG with Prostate Cancer
In prostate cancer, immunohistochemical analysis revealed minimal expression of TPBG in normal prostate tissue, scattered low-level expression in BPH tissue, and mostly localized in basal cells, while TPBG expression was significantly increased in prostate cancer cells. Trovax has also been clinically studied in prostate cancer patients. The effects of Trovax alone or Trovax in combination with granulocyte-macrophage colony-stimulating factor (GM-CSF) showed no serious toxic effects. Patients in the TroVax group had a prolonged disease progression compared to the group with GM-CSF [11].
3.3 TPBG with Kidney Cancer
In kidney cancer, statistical analysis of TPBG and CXCR4 co-expression in 72 cases of renal clear cell carcinoma showed a positive correlation between TPBG and both renal clear cell pathological grade and clinical stage. Immunofluorescence synchronization showed co-expression localization of TPBG and CXCR4. MVA-5T4 was also used in the treatment of metastatic kidney cancer. Different clinical trials were conducted, suggesting that MVA-5T4 inoculation induced a 5T4/TPBG antibody response, which was correlated with the prognosis of cancer patients. Patients treated with MVA-5T4 had mounted a positive 5T4 antibody response than healthy individuals [12, 13].
3.4 TPBG with Ovarian and Metastatic Cancer
In ovarian and metastatic cancers, immunohistochemical analysis showed that high expression of TPBG, implying that TPBG can be used as an indicator of poor prognosis in ovarian cancer. In an analysis of 72 ovarian cancers, there was a close relationship between TPBG expression and disease stages [14, 15]. Furthermore, as noted by other studies, there is an effective antitumor activity of 5T4-specific CAR-T cells against ovarian cancer cells in vitro and xenotransplanted tumors in vivo. 5T4 CAR-T can elicit lytic cytotoxicity in targeted tumor cells, in addition to the secretion of cytotoxic cytokines, including IFN-γ, IL-2, and GM-CSF. As such, TPBG/5T4 is likely another valid target in ovarian cancer besides to HER2.
3.5 TPBG with B-Cell Acute Lymphoblastic Leukemia (ALL)
Studies in B-cell acute lymphoblastic leukemia (ALL) have shown that TPBG is expressed in high risk of relapse childhood pre-B acute lymphoblastic leukemia and is associated with a more invasive and chemotactic phenotype. Gene expression profiling of 85 diagnostic pre-B-ALL bone marrow samples revealed higher TPBG transcript levels in cytogenetic high-risk subgroups of patients. Existing studies already demonstrated a role for the TPBG in the regulation of CXCL12/CXCR4. It is speculated that TPBG may stabilize the expression of CXCL12/CXCR4 on the cell membrane and function to facilitate chemotaxis. In short, TPBG might exert a joint influence upon tumorigenesis via mediating CXCL12/CXCR4 axis.
4. TPBG Clinical Trials for Drug Development
Currently, many pharmaceutical companies are conducting clinical trials on drugs targeting TPBG, including Pfizer Inc., Oxford Biomedica Plc., Wales Cancer Research Centre, Genmab A/S, NeoTX Therapeutics Ltd., Aptevo Therapeutics, Inc., Alligator Bioscience AB, Chiome Bioscience, Inc., Synthon BV etc. Different approaches are available for the immunologic targeting of TPBG, including monoclonal antibodies, bispecific antibodies, trispecific antibodies, ADCs, and tumor vaccines, mainly for cancer treatment, such as breast cancer, renal cell carcinoma, bladder cancer, non-small cell lung cancer (Table 1).
As mentioned earlier, data from several pharmaceutical companies have revealed that TPBG drugs are strongly associated with clinical benefit, demonstrating that TPBG antibodies or vaccines have significant value in cancer treatment. Thus, TPBG is expected to be alternative potential target for antitumor immunotherapy!
|
Drugs
|
Alias
|
Target
|
Mechanism of action
|
Drug Type
|
Indications
|
Institutes
|
R&D Status
|
|
OXB-301
|
Modified vaccinia Ankara 5T4; MVA-5T4; MVA-h 5T4; OBA 1 cancer vaccine; SAR-109659; rV-5T4-VAC; TroVax; MVA 5T4; Recombinant modified vaccinia Ankara 5T4 vaccine; 5T4 cancer vaccine
|
5T4
|
T-lymphocyte stimulator; 5T4 modulator
|
Therapeutic vaccines
|
Renal cell carcinoma
|
Oxford Biomedica Plc; Wales Cancer Research Centre; University of Cardiff; University College London
|
Clinical Phase 3
|
|
GEN-1044
|
GEN 1044; DuoBody-CD3x5T4
|
5T4; CD3
|
CD3 regulator; 5T4 regulator
|
Bispecific antibodies
|
Bladder cancer; esophageal cancer; tumor metastasis; non-small cell lung cancer; prostate cancer; triple-negative breast cancer; uterine cancer; tumor; solid tumor
|
Genmab A/S
|
Clinical Phase 2
|
|
Naptumomab estafenatox
|
TTS-CD3; Anyara; TTS CD3; ABR-217620
|
5T4
|
T-lymphocyte stimulator; 5T4 modulator
|
Fusion proteins; antibody-coupled toxins
|
Non-small cell lung cancer; solid tumors
|
NeoTX Therapeutics Ltd.
|
Clinical Phase 2
|
|
ALG.APV-527
|
ATOR-1016; ADC-1016
|
5T4;4-1BB
|
4-1BB agonist; 5T4 modulator
|
Bispecific antibodies
|
Solid tumors; tumors
|
Aptevo Therapeutics, Inc.; Alligator Bioscience AB
|
Clinical Phase 1
|
|
ASN-004
|
ASN-004; Dolaflexin® ADC - Asana BioSciences; Fleximer® - ADC - Endo Pharmaceuticals/Mersana Therapeutics
|
5T4
|
5T4 regulator
|
ADC; monoclonal antibody
|
Solid tumors; breast cancer; colorectal cancer; metastatic breast cancer; tumors; non-small cell lung cancer; ovarian cancer
|
Asana BioSciences LLC
|
Clinical Phase 1
|
|
CBA-1535
|
CBA-1535; Tb535H
|
5T4; CD3
|
CD3 inhibitor; 5T4 modulator
|
Tri-Specific Antibodies
|
Tumors
|
Chiome Bioscience, Inc.
|
Clinical Phase 1
|
|
IBR854
|
Conjugated Antibody Redirecting ready-to-use allogeneic NK cells; CAR-raNK
|
5T4
|
Natural killer cell replacement; 5T4 modulator
|
CAR-NK
|
Solid tumors
|
Infinera (Hangzhou) Biopharmaceutical Co.
|
Clinical Phase 1
|
|
SYD-1875
|
Anti-5T4 antibody drug conjugate; SYD 1875
|
5T4
|
5T4 regulator
|
ADC; monoclonal antibody
|
Solid tumors; tumors
|
Synthon BV; Byondis BV
|
Clinical Phase 1
|
|
Anti-TPBG antibody-drug conjugate(XDCExplorer)
|
anti-TPBG antibody-drug conjugate(XDCExplorer)
|
5T4
|
5T4 regulator
|
ADC; monoclonal antibody
|
Tumors
|
Kaihui Technology Development (Shanghai) Co.
|
Preclinical
|
|
DF-7001
|
DF7001
|
5T4; CD16a
|
5T4 modulator; CD16a antagonist
|
Tri-Specific Antibodies
|
Inflammation; tumors
|
Dragonfly Therapeutics, Inc.; Gilead Sciences, Inc.
|
Preclinical
|
|
DM004
|
-
|
5T4; MerTK
|
5T4 modulator; MerTK inhibitor
|
ADC; monoclonal antibody
|
Solid tumors
|
Sidao Pharmaceutical Technology (Suzhou) Co.
|
Preclinical
|
|
INBRX-130
|
INBRX-130
|
5T4; CD3
|
CD3 inhibitor; 5T4 modulator
|
Bispecific antibodies
|
Tumors
|
Inhibrx, Inc.
|
Preclinical
|
|
MEDI-0641
|
MEDI-0641
|
5T4
|
5T4 regulator
|
ADC; monoclonal antibody
|
Solid tumors
|
MedImmune LLC
|
Preclinical
|
|
Tb-535
|
Tb535; Tb-535H
|
5T4
|
5T4 regulator
|
Tri-Specific Antibodies
|
Solid tumors
|
Biotecnol Ltd.
|
Preclinical
|
|
5T4-5T4-CD3
|
5T4-5T4-CD3
|
5T4; CD3
|
CD3 inhibitor; 5T4 modulator
|
Bispecific antibodies
|
/
|
/
|
In drug discovery
|
|
5T4-X-CD3
|
5T4-X-CD3
|
5T4; CD3
|
CD3 inhibitor; 5T4 modulator
|
Monoclonal antibodies
|
/
|
/
|
In drug discovery
|
|
CME-548 (Oxford Biomedica Plc)
|
CME-548
|
5T4
|
-
|
ADC; monoclonal antibody
|
/
|
/
|
In preclinical
|
|
CTM-041
|
CTM041; CTM 041
|
5T4; CD40
|
CD40 inhibitor; 5T4 modulator
|
Bispecific antibodies
|
/
|
/
|
/
|
Table 1: Progress of TPBG clinical studies
To assist researchers or pharmaceutical companies in their research on TPBG in tumors or other diseases, CUSABIO presents TPBG active protein product (CSB-MP024093HUb0; CSB-MP024093MOV) to support your research on the mechanism of TPBG or its potential clinical value.
● Recombinant Human Trophoblast glycoprotein(TPBG),partial (Active)
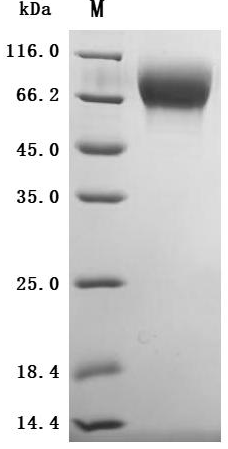
High Purity Validated by SDS-PAGE
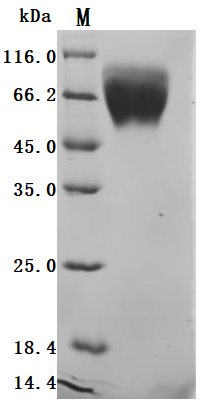
The purity was greater than 95% as determined by SDS-PAGE.(Tris-Glycine gel) Discontinuous SDS-PAGE (reduced) with 5% enrichment gel and 15% separation gel.
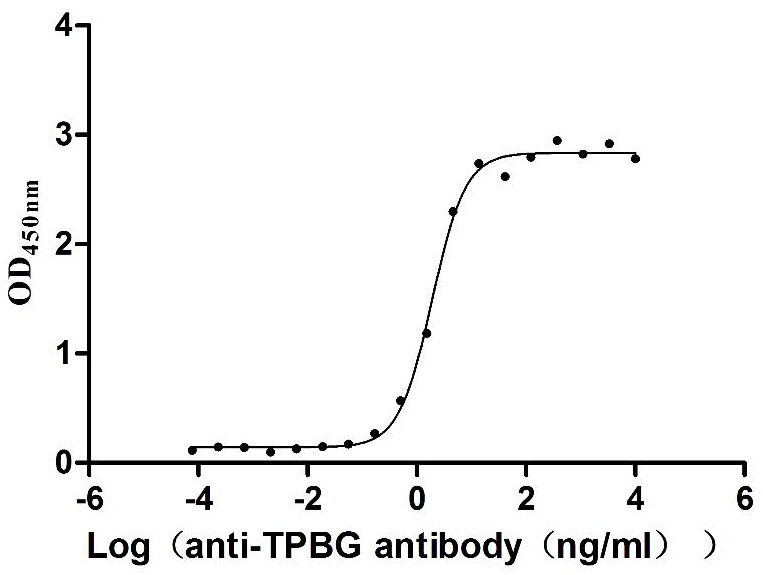
Excellent Bioactivity Validated by Functional ELISA
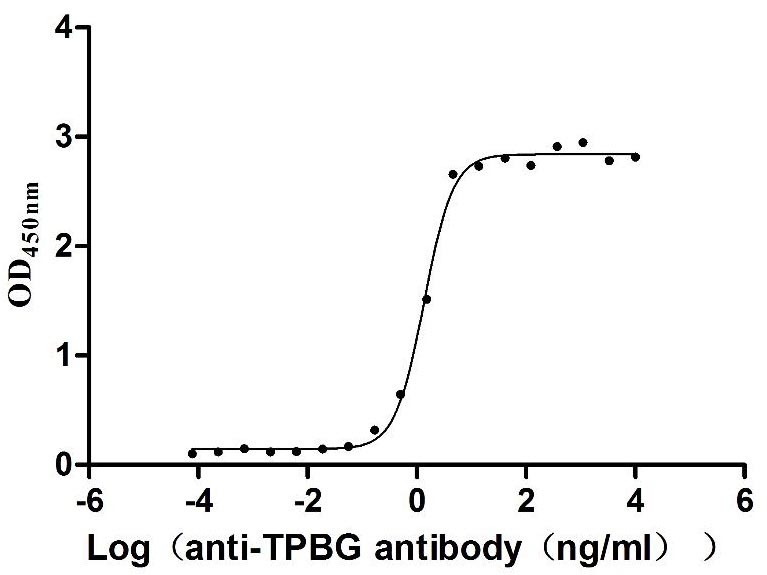
Immobilized Human TPBG at 2 μg/mL can bind Anti-TPBG recombinant antibody (CSB-RA024093MA1HU), the EC50 is 1.230-1.519 ng/mL.
References
[1] Xu, Y., Morales, A.J., Cargill, M.J. et al. Preclinical development of T-cell receptor-engineered T-cell therapy targeting the 5T4 tumor antigen on renal cell carcinoma. Cancer Immunol Immunother 68, 1979-1993 (2019).
[2] Zhao, Yuguang, et al. "Structural insights into the inhibition of Wnt signaling by cancer antigen 5T4/Wnt-activated inhibitory factor 1." Structure 22.4 (2014): 612-620.
[3] Stern, Peter L., and Richard Harrop. "5T4 oncofoetal antigen: an attractive target for immune intervention in cancer. "Cancer Immunology, Immunotherapy 66.4 (2017): 415-426.
[4] Spencer, Helen L., et al. "Role of TPBG (trophoblast glycoprotein) antigen in human pericyte migratory and angiogenic activity. "Arteriosclerosis, Thrombosis, and Vascular Biology 39.6 (2019): 1113-1124.
[5] Harrop, Richard, Eric O'Neill, and Peter L. Stern. "Cancer stem cell mobilization and therapeutic targeting of the 5T4 oncofetal antigen. " Therapeutic advances in vaccines and immunotherapy 7 (2019): 2515135518821623.
[6] Castro, Fernanda V., et al. "5T4 oncofetal antigen is expressed in high risk of relapse childhood pre-B acute lymphoblastic leukemia and is associated with a more invasive and chemotactic phenotype." Leukemia 26.7 (2012): 1487-1498.
[7] Stern, Peter L., et al. "Understanding and exploiting 5T4 oncofoetal glycoprotein expression. "Seminars in Cancer Biology. vol. 29. Academic Press, 2014.
[8] Tsuboi, Akio. "LRR-containing oncofetal trophoblast glycoprotein 5T4 shapes neural circuits in olfactory and visual systems. "Frontiers in Molecular Neuroscience 13 (2020): 581018.
[9] Scurr, Martin, et al. "Escalating Regulation of 5T4-Specific IFN-γ+ CD4+ T Cells Distinguishes Colorectal Cancer Patients from Healthy Controls and Provides a Target for In Vivo TherapyAnti-5T4 T-cell Responses in Advanced Cancer." Cancer immunology research 1.6 (2013): 416-425.
[10] Harrop, Richard, et al. "Vaccination of colorectal cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) induces immune responses which correlate with disease control: a phase I/II trial." Clinical Cancer Research 12.11 (2006): 3416-3424.
[11] Amato, Robert J., et al. "Vaccination of prostate cancer patients with modified vaccinia ankara delivering the tumor antigen 5T4 (TroVax): a phase 2 trial." Journal of immunotherapy 31.6 (2008): 577-585.
[12] Elkord, Eyad, et al. "5T4 as a target for immunotherapy in renal cell carcinoma." Expert Review of Anticancer Therapy 9.12 (2009): 1705-1709.
[13] Griffiths, Richard W., et al. "Expression of the 5T4 oncofoetal antigen in renal cell carcinoma: a potential target for T-cell-based immunotherapy." British journal of cancer 93.6 (2005): 670-677.
[14] Owens, Gemma L., et al. "Preclinical assessment of CAR T-cell therapy targeting the tumor antigen 5T4 in ovarian cancer." journal of Immunotherapy ( Hagerstown, Md.: 1997) 41.3 (2018): 130.
[15] Wan, Y. Louise, et al. "A systems biology approach to understanding the function of 5T4 oncofetal glycoprotein in ovarian cancer and the preclinical effectiveness of a 5T4 antibody drug conjugate." The Lancet 389 (2017): S100.
[16] Castro, Fernanda V., et al. "5T4 oncofetal antigen is expressed in high risk of relapse childhood pre-B acute lymphoblastic leukemia and is associated with a more invasive and chemotactic phenotype." Leukemia 26.7 (2012): 1487-1498.
[17] Starzynska, T., et al. "Prognostic significance of 5T4 oncofetal antigen expression in colorectal carcinoma." British journal of cancer 69.5 (1994): 899-902.
CUSABIO team. TPBG/5T4: the Oncofetal Protein, an Appealing Target for ADC or Vaccine Therapeutics in Tumors!. https://www.cusabio.com/c-21103.html






Comments
Leave a Comment