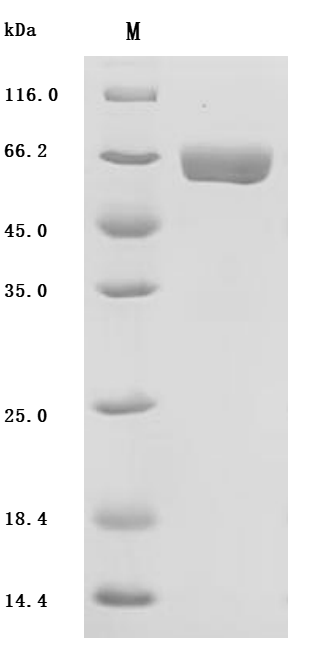
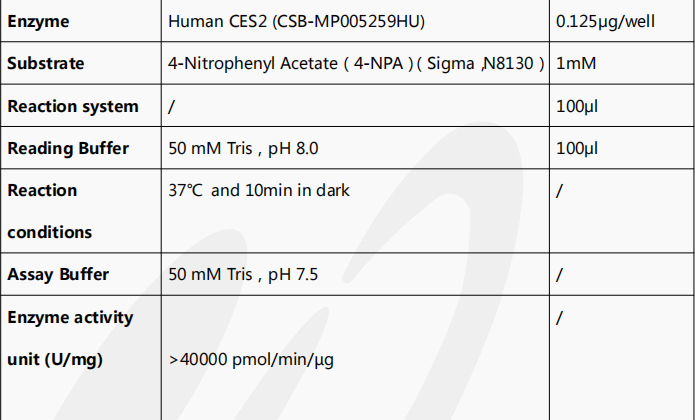
Human CES2 protein is the recombinant human-derived CES2 protein, expressed by HEK293 cells, with a C-terminal 10xHis labeled tag. Its target gene corresponds to the 27-559aa of the human CES2. It has a high purity, up to 95% as determined by SDS-PAGE, and a low endotoxin level, less than 1.0 EU/ug as measured by the LAL method. It is an active protein as validated by its ability to hydrolyze p-nitrophenylacetate, with a specific activity greater than 40000 pmol/min/µg.
The human CES2 protein is a member of the carboxylesterase enzyme family, which plays a crucial role in the hydrolysis of ester and amide bonds in various substrates, including prodrugs and xenobiotics. CES2 is primarily expressed in the liver and small intestine, where it is involved in the metabolism of certain drugs, especially ester drugs, by converting them into their active forms, thereby enhancing the bioavailability of the drug [1][2]. This enzymatic activity is vital for the pharmacokinetics of these medications, influencing their efficacy and safety profiles.
In addition to its role in drug metabolism, CES2 has been implicated in lipid metabolism and homeostasis. Studies have shown that CES2 helps prevent liver steatosis by modulating lipolysis and endoplasmic reticulum stress, particularly under conditions of metabolic stress such as obesity and diabetes [3][4]. The expression of CES2 is downregulated in these conditions, which may contribute to the development of non-alcoholic fatty liver disease (NAFLD) [4]. The enzyme's activity can be influenced by various factors, including genetic polymorphisms and environmental conditions, which may lead to variability in drug metabolism among individuals [5].
References:
[1] M. Ross, A. Borazjani, R. Wang, J. Crow, & S. Xie. Examination of the carboxylesterase phenotype in human liver, Archives of Biochemistry and Biophysics, vol. 522, no. 1, p. 44-56, 2012. https://doi.org/10.1016/j.abb.2012.04.010
[2] A. Basit, N. Neradugomma, C. Wolford, P. Fan, B. Murray, R. Takahashi, et al. Characterization of differential tissue abundance of major non-cyp enzymes in human, Molecular Pharmaceutics, vol. 17, no. 11, p. 4114-4124, 2020. https://doi.org/10.1021/acs.molpharmaceut.0c00559
[3] Y. Li, M. Zalzala, K. Jadhav, Y. Xu, T. Kasumov, L. Yin, et al. Carboxylesterase 2 prevents liver steatosis by modulating lipolysis, endoplasmic reticulum stress, and lipogenesis and is regulated by hepatocyte nuclear factor 4 alpha in mice, Hepatology, vol. 63, no. 6, p. 1860-1874, 2016. https://doi.org/10.1002/hep.28472
[4] M. Ruby, J. Massart, D. Hunerdosse, M. Schönke, J. Correia, S. Louie, et al. Human carboxylesterase 2 reverses obesity-induced diacylglycerol accumulation and glucose intolerance, Cell Reports, vol. 18, no. 3, p. 636-646, 2017. https://doi.org/10.1016/j.celrep.2016.12.070
[5] J. Liu, X. Shang, S. Huang, Y. Xu, J. Lu, Y. Zhang, et al. Construction and characterization of crispr/cas9 knockout rat model of carboxylesterase 2a gene, Molecular Pharmacology, vol. 100, no. 5, p. 480-490, 2021. https://doi.org/10.1124/molpharm.121.000357