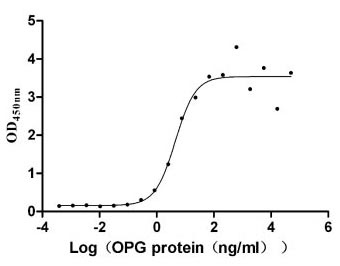
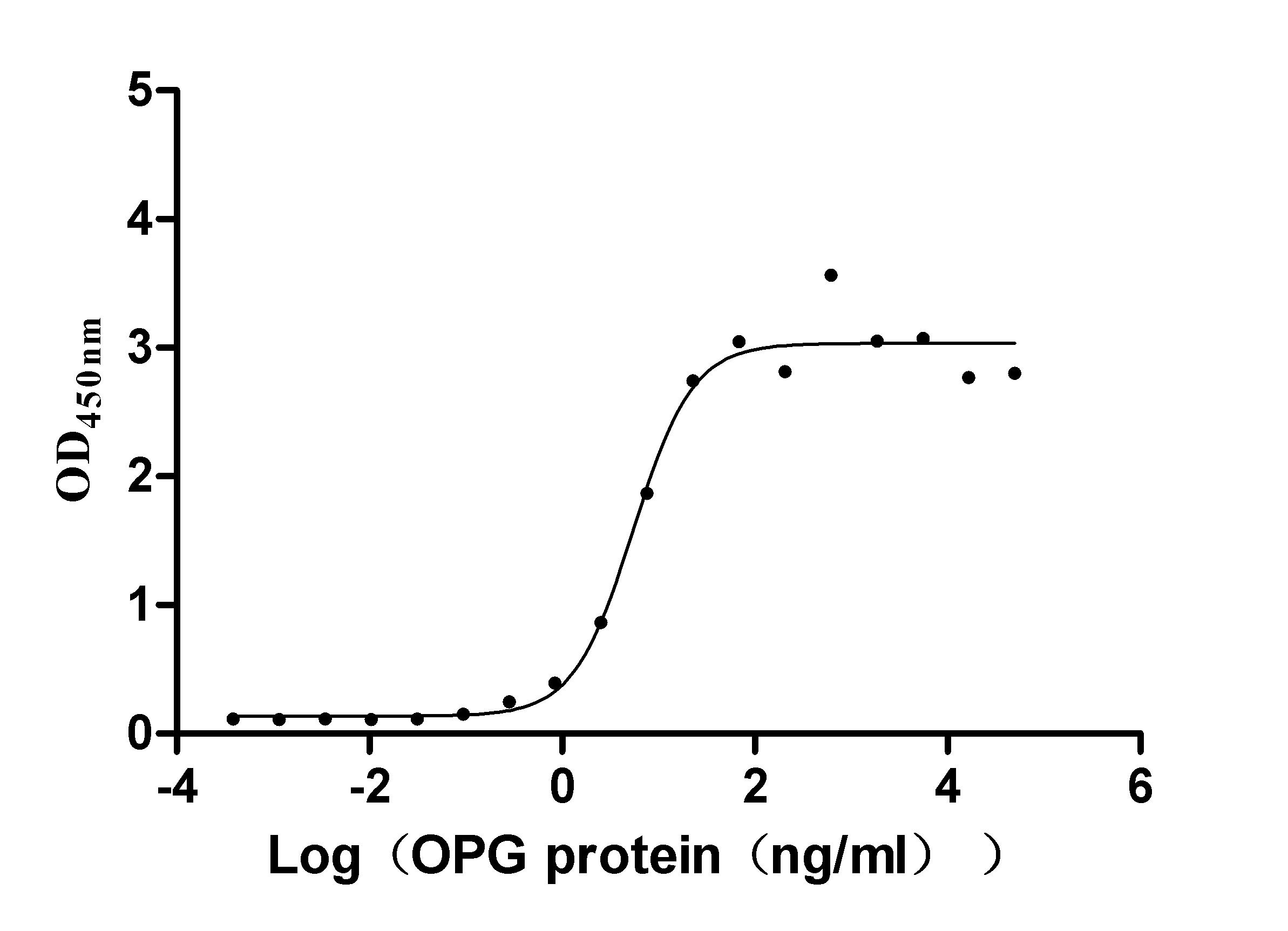
The production of the recombinant human TNFRSF11B protein involves expressing a plasmid containing the gene coding for residues 22-401 of TNFRSF11B in mammalian cells. The TNFRSF11B protein is fused with a C-terminal hFc-Flag-tag. SDS-PAGE analysis shows the TNFRSF11B protein has a purity of over 90%, and endotoxin content is confirmed to be below 1.0 EU/μg using the LAL method. ELISA confirms the TNFRSF11B protein's biological activity, with specific binding to the TNFSF11 (CSB-MP023986HU1(F2)). The EC50 is 2.651-7.646 ng/mL.
Human TNFRSF11B, also known as osteoprotegerin (OPG), is a crucial glycoprotein important for bone metabolism and the regulation of osteoclastogenesis. It is primarily produced by osteoblasts, although it is also expressed in various tissues, including the heart and blood vessels [1]. OPG functions primarily as a decoy receptor for the RANKL, inhibiting its interaction with RANK on osteoclasts, thereby preventing osteoclast differentiation and activation, which are essential processes in bone resorption [2][3].
Mutations in the TNFRSF11B gene can lead to various bone disorders, including juvenile Paget's disease (JPD), which is characterized by increased bone turnover, deformities, and fractures [4][5]. In JPD, loss-of-function mutations in TNFRSF11B result in the absence of OPG, leading to uncontrolled osteoclast activity and excessive bone resorption [6]. Furthermore, polymorphisms in the TNFRSF11B gene have been associated with susceptibility to osteoporosis and low bone mineral density, highlighting its importance in maintaining bone health [7][8].
Recent studies have also indicated that TNFRSF11B may have implications beyond bone metabolism. Elevated levels of OPG have been linked to inflammatory conditions and certain cancers, suggesting a potential role in tumor biology and inflammation [9][10]. The co-amplification of TNFRSF11B with oncogenes like MYC has been associated with poor prognosis in gastric cancer, indicating its relevance in cancer progression [9]. Additionally, OPG's ability to inhibit apoptosis may contribute to its role in cancer biology, further complicating its functional profile [10].
References:
[1] J. Xue, X. Zhan, W. Wang, Y. Yan, & C. Lei. Opg rs2073617 polymorphism is associated with upregulated opg protein expression and an increased risk of intervertebral disc degeneration, Experimental and Therapeutic Medicine, vol. 12, no. 2, p. 702-710, 2016. https://doi.org/10.3892/etm.2016.3342
[2] Y. Bu, Y. He, H. Zhou, W. Liu, D. Peng, A. Tang, et al. Insulin receptor substrate 1 regulates the cellular differentiation and the matrix metallopeptidase expression of preosteoblastic cells, Journal of Endocrinology, vol. 206, no. 3, p. 271-277, 2010. https://doi.org/10.1677/joe-10-0064
[3] K. Cawley, N. Bustamante-Gomez, A. Guha, R. MacLeod, J. Xiong, I. Gubrij, et al. Local production of osteoprotegerin by osteoblasts suppresses bone resorption, Cell Reports, vol. 32, no. 10, p. 108052, 2020. https://doi.org/10.1016/j.celrep.2020.108052
[4] F. Saki, Z. Karamizadeh, S. Nasirabadi, S. Mumm, W. McAlister, & M. Whyte. Juvenile Paget's disease in an Iranian kindred with vitamin D deficiency and novel homozygous TNFRSF11B mutation, Journal of Bone and Mineral Research, vol. 28, no. 6, p. 1501-1508, 2013. https://doi.org/10.1002/jbmr.1868
[5] A. Daroszewska, L. Hocking, F. McGuigan, B. Langdahl, M. Stone, T. Cundy, et al. Susceptibility to paget's disease of bone is influenced by a common polymorphic variant of osteoprotegerin, Journal of Bone and Mineral Research, vol. 19, no. 9, p. 1506-1511, 2004. https://doi.org/10.1359/jbmr.040602
[6] P. Salmon. Loss of chaotic trabecular structure in opg-deficient juvenile paget's disease patients indicates a chaogenic role for opg in nonlinear pattern formation of trabecular bone, Journal of Bone and Mineral Research, vol. 19, no. 5, p. 695-702, 2004. https://doi.org/10.1359/jbmr.040210
[7] G. Beyens, A. Daroszewska, F. Freitas, E. Fransén, F. Vanhoenacker, L. Verbruggen, et al. Identification of sex-specific associations between polymorphisms of the osteoprotegerin gene, tnfrsf11b, and paget's disease of bone, Journal of Bone and Mineral Research, vol. 22, no. 7, p. 1062-1071, 2007. https://doi.org/10.1359/jbmr.070333
[8] C. Vidal, R. Formosa, & A. Xuereb-Anastasi. Functional polymorphisms within the tnfrsf11b (osteoprotegerin) gene increase the risk for low bone mineral density, Journal of Molecular Endocrinology, vol. 47, no. 3, p. 327-333, 2011. https://doi.org/10.1530/jme-11-0067
[9] X. Wang, Y. Liu, D. Shao, Q. Zhou, Z. Dong, Y. Sun, et al. Recurrent amplification of myc and tnfrsf11b in 8q24 is associated with poor survival in patients with gastric cancer, Gastric Cancer, vol. 19, no. 1, p. 116-127, 2015. https://doi.org/10.1007/s10120-015-0467-2
[10] R. Ito, H. Nakayama, K. Yoshida, K. Kuraoka, J. Motoshita, N. Oda, et al. Expression of osteoprotegerin correlates with aggressiveness and poor prognosis of gastric carcinoma, Virchows Archiv, vol. 443, no. 2, p. 146-151, 2003. https://doi.org/10.1007/s00428-003-0845-8