What Are Osteoclasts?
Osteoclasts (OCs) are multinucleated giant cells with the function of bone resorption. Multinucleated giant cells are formed by the fusion of mononuclear macrophages differentiated from myeloid hematopoietic stem cells. And osteoclasts play an important role in the development and formation of the bone skeleton, in synergy with osteoblasts.
What Is Osteoclast Differentiation?
Early immature proliferative mononuclear phagocytic cells, also called osteoclast precursors, enter the blood circulation under the action of chemical factors and then enter the bone structure cavity under the action of signal factors released by basal multicellular units. Under the stimulation of various chemical factors, transcription factors, cytokines, and other signaling factors, they are fused into multinucleated cells and finally activated into osteoclasts. This process is called osteoclast differentiation.
The Mechanism of Osteoclast Differentiation
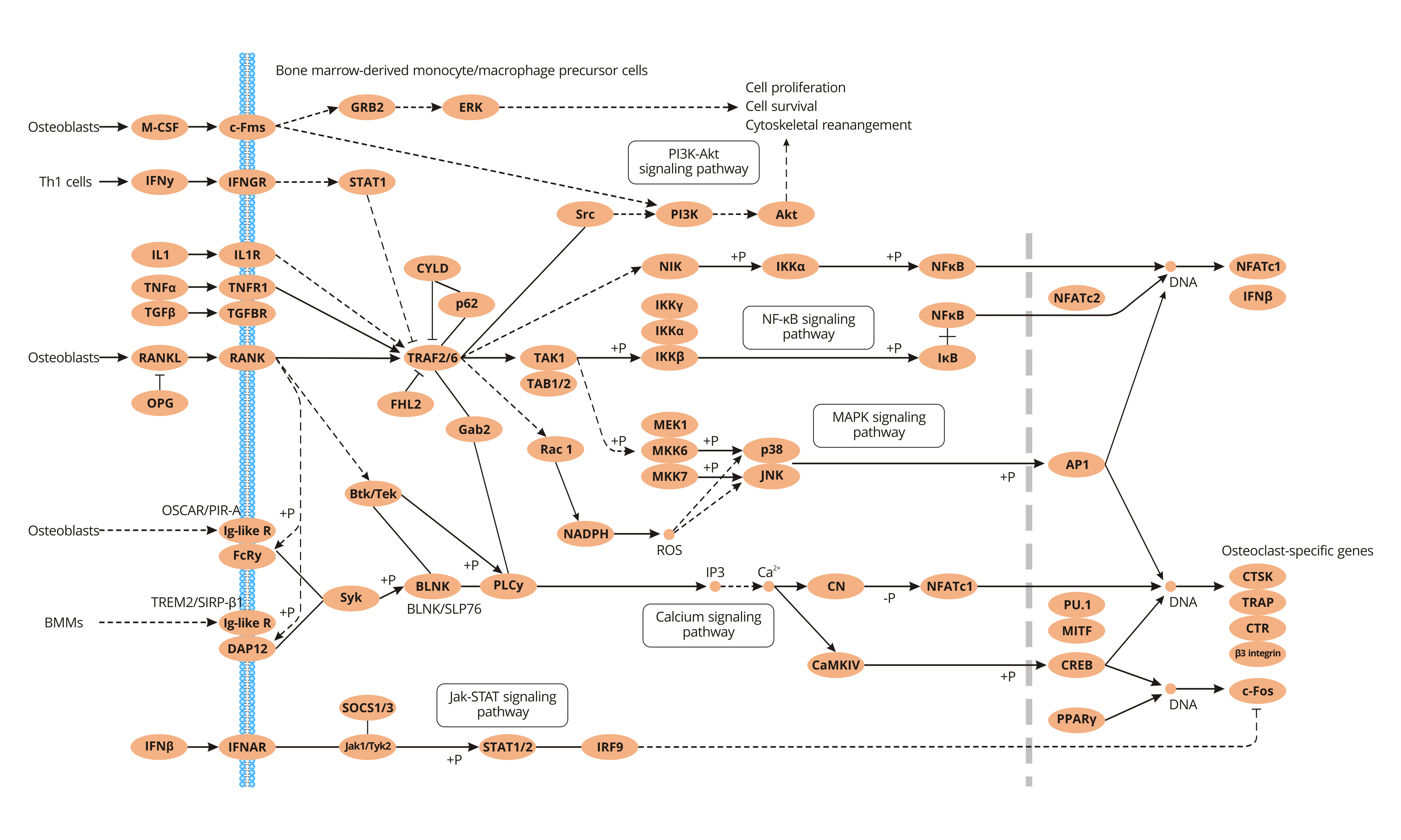
Two essential cytokines macrophage colony-stimulating factor (M-CSF) and the receptor activator of nuclear factor-κB ligand (RANKL) regulate osteoclast differentiation and activity.
M-CSF is a key player in promoting the proliferation and survival of osteoclast precursor cells. M-CSF binds to and phosphorylates c-Fms. c-Fms phosphorylation activates itself and then recruits some downstream targets such as phosphatidylinositol 3-kinase (PI3K), Akt, ERK, and Grb2, triggering the ERK and PI3K/Akt pathways that enhance osteoclast precursor cells proliferation and survival.
With the aid of M-CSF, osteoclast precursor cells express RANK (receptor activator of NF-kappa B), which binds to RANKL. RANKL-RANK recruits TRAFs (tumor necrosis factor receptor-associated factors) to the RANK' cytoplasmic region, where TRAKs (TRAF1, TRAF2, TRAF3, TRAF5, and TRAF6) binds to RANK. Among TRAFs, TRAF6 is the most important for osteoclast formation. TRAF6 binds to RANK, initiating and transmitting the differentiation signal of osteoclasts through the JNK (c-Jun N-terminal kinase) pathway, NF-Kappa B pathway, and Akt pathway. TRAF2 and TRAF5 link to RANK, which activates c-Jun N-terminal kinase (JNK). JNK induces c-Jun/Fos-activated protein 1 activation, which regulates c-Fos expression, promoting proliferation and differentiation of osteoclast precursors. TRAF6 binds to RANK to activate phosphatidylinositol-3-kinase (PI-3K), which in turn activates protein kinase B (PKB), Akt, and NF-Kappa B, increasing c-Fos expression. c-Fos combines with the nuclear factor of activated T cells (NFATc1), initiating transcription of osteoclast-specific genes and inducing osteoclast precursor differentiation into mature osteoclasts. Except for the self-regulatory mechanism, elevated bone resorption and blood calcium levels induced by osteoclast maturation and activation also play a regulatory role in the pathway loop. Activation of calcium signaling also stimulates NFATc1 expression.
Regulation of Osteoclast Differentiation
OPG (osteoprotegerin) exerts its role in the form of osteoblast/stromal cell paracrine secretion. OPG competitively binds to OPGL (or RANKL), which blocks OPGL binding to RANK. The combination between OPG and OPGL inhibits RANKL/RANK pathway, repressing osteoclast differentiation and maturation and prevent osteoclast cells from excessively growing. Besides, OPG can also bind to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and inhibit TRAIL-induced apoptosis.
The RANKL/OPG ratio is related to the strength of osteoclast differentiation. If the ratio decreases, the RANKL on the surface of osteoblasts is all competitively bound by OPG, rather than binding to RANK on the osteoclast precursor, inhibiting differentiation of osteoclasts. If the ratio excessively increases, OPG is difficult to antagonize the binding of RANKL and RANK, resulting in increased osteoclastogenesis and enhanced bone resorption capacity. Therefore, keeping the RANKL/OPG ratio at a certain range is important for maintaining osteoclast differentiation and bone metabolism balance.





