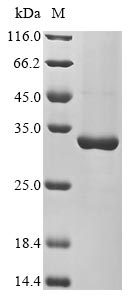
Recombinant Arabidopsis thaliana Heat shock protein 21 (HSP21) is produced in an E. coli expression system, spanning the full length of the mature protein from amino acids 44 to 227. The protein includes an N-terminal 10xHis-tag and a C-terminal Myc-tag, which streamline purification and detection processes. SDS-PAGE analysis confirms purity greater than 90%, suggesting this preparation should deliver reliable results for research applications.
Heat shock protein 21 (HSP21) in Arabidopsis thaliana appears to be a chloroplast-localized protein that may play a crucial role in how plants respond to heat stress. This protein belongs to the small heat shock protein family, which works to stabilize and refold denatured proteins, helping maintain cellular balance. Research into HSP21 could be vital for understanding plant stress responses and how they adapt to challenging conditions.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Although the protein covers the full-length mature sequence (44-227aa), several factors raise concerns: 1) The dual tagging system (N-terminal 10xHis and C-terminal Myc) may significantly interfere with proper folding - the C-terminal Myc tag is particularly problematic as it could disrupt the conserved C-terminal region essential for oligomerization and client protein binding in small heat shock proteins; 2) The E. coli expression system lacks chloroplast-specific factors (redox environment, chaperones) necessary for proper folding of plant chloroplastic proteins; 3) HSP21 requires correct oligomerization and disulfide bond formation for function, which may be compromised by the tags and prokaryotic expression. The >90% purity indicates low contaminants, but doesn't guarantee proper quaternary structure formation. Without validation through circular dichroism, thermal stability assays, and chaperone activity tests, the protein's functional status remains uncertain.
1. In Vitro Protein-Protein Interaction Studies
This dual-tagged HSP21 can be used for interaction studies only if proper folding and oligomerization are verified. The tags may cause steric hindrance or non-specific binding - the C-terminal Myc tag could block natural interaction interfaces. Before pull-down or co-IP experiments, validate the protein's ability to form functional oligomers and interact with known client proteins. Include tag-only controls and consider using tag-free protein for biologically relevant results.
2. Antibody Development and Validation
The recombinant protein can generate antibodies, but the dual tags may dominate the immune response, resulting in antibodies that primarily recognize tags rather than HSP21-specific epitopes. If misfolded, antibodies may not recognize native chloroplastic HSP21. For reliable antibodies, validate using tag-free protein and confirm recognition of endogenous HSP21 in plant tissues. The Myc tag provides a detection control but doesn't ensure utility for native protein studies.
3. Biochemical Characterization of Heat Shock Response
This protein is suitable for biophysical characterization only if the tags are removed and proper oligomerization is confirmed. The dual tags may alter thermal stability and oligomeric state measurements. For circular dichroism or dynamic light scattering, use a tag-free protein to obtain meaningful data. The E. coli expression system may not replicate chloroplast-specific modifications affecting stability.
4. Comparative Plant Stress Biology Research
The recombinant HSP21 can serve as a reference for comparative studies only if properly folded. The tags may introduce structural artifacts that complicate cross-species comparisons. Validate that the protein's structural and functional properties match those of native HSP21 before comparative analyses. Tag removal is recommended for evolutionary studies.
5. Chloroplast Proteomics and Pathway Analysis
This protein can work as a positive control in proteomics if the tags don't interfere with detection. However, the Myc tag may cause dominance in mass spectrometry analysis. For accurate quantification, use tag-free protein or validate that tag presence doesn't affect detection sensitivity and specificity in complex mixtures.
Final Recommendation & Action Plan
To ensure reliable results, first validate the protein's folding and functionality: remove both tags via proteolytic cleavage (if cleavage sites are available) and purify the tag-free protein; characterize secondary structure by circular dichroism (expecting high β-sheet content typical of sHSPs); test oligomerization state via size-exclusion chromatography with multi-angle light scattering; and validate chaperone activity through thermal aggregation protection assays using client proteins like citrate synthase. For applications requiring native-like behavior, always use tag-free protein. If tags must remain, include rigorous controls: tag-only proteins for interaction studies, stress-conditioned plant extracts for antibody validation, and known HSP21 standards for comparative analyses. The E. coli expression system is cost-effective but may require additional refolding steps to achieve functional protein.