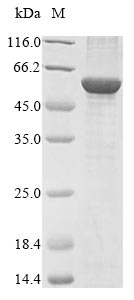
Recombinant Bovine NADPH:adrenodoxin oxidoreductase, mitochondrial (FDXR) is expressed in E. coli and spans the full length of the mature protein, covering amino acids 33 to 492. The product comes with an N-terminal 10xHis tag, which makes purification and detection more straightforward. SDS-PAGE analysis confirms the protein reaches greater than 85% purity—this level appears adequate for most research applications.
NADPH:adrenodoxin oxidoreductase, mitochondrial (FDXR) plays a critical role in electron transfer processes within the mitochondria. The protein acts as an essential bridge in steroidogenesis, moving electrons from NADPH to adrenodoxin. Its importance in steroid hormone synthesis and mitochondrial electron transport pathways likely makes it valuable for biochemical and physiological research.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Bovine FDXR is a complex mitochondrial protein that requires proper folding to bind FAD and NADPH cofactors and facilitate electron transfer to adrenodoxin. While E. coli can express soluble recombinant proteins, FDXR's multiple domain structure and cofactor incorporation present significant challenges. The bacterial system may produce folded protein, but likely lacks the specific chaperones and environment for optimal FAD incorporation and native conformation. The protein may have some structural integrity, but its enzymatic activity is uncertain without validation.
1. Protein-Protein Interaction Studies with Adrenodoxin and Cytochrome P450 Enzymes
This application carries substantial risk. If correctly folded with incorporated FAD, the protein could yield meaningful interaction data with adrenodoxin. However, if misfolded or lacking proper cofactor binding, it may exhibit non-specific binding or fail to interact authentically, leading to misleading results about the electron transfer complex formation. Protein-protein interactions in this electron transfer system require precise conformational alignment and functional cofactors. Results would be unreliable without prior validation of proper folding and activity.
2. Antibody Development and Validation
This recombinant FDXR serves as an excellent immunogen for generating specific antibodies. The full-length mature protein sequence provides comprehensive epitope coverage. The His-tag facilitates purification and immunization procedures. Resulting antibodies will be valuable for detecting FDXR in bovine tissues regardless of the protein's enzymatic activity.
3. Biochemical Characterization and Enzyme Kinetics Analysis
This is the critical first step to assess protein quality. Techniques like UV-visible spectroscopy can detect FAD incorporation, while activity assays with NADPH can determine enzymatic function. Size exclusion chromatography and thermal shift assays can evaluate oligomeric state and stability. These studies directly address the folding and activity uncertainties and provide essential data for evaluating the protein's functional capabilities.
4. Reconstitution of Mitochondrial Electron Transfer Systems
Functional reconstitution requires fully active components. If the FDXR demonstrates proper FAD binding and electron transfer capability, it could be used in reconstitution studies. However, without activity confirmation, such experiments would yield meaningless results and waste valuable cytochrome P450 components.
Final Recommendation & Action Plan
The critical unknown of this recombinant FDXR's functional status mandates a sequential validation approach before committing to complex experiments. The immediate and essential first step is Application 3 (Biochemical Characterization) to rigorously test FAD incorporation via absorbance spectroscopy and validate electron transfer activity using established adrenodoxin reduction assays. If the protein demonstrates proper cofactor binding and enzymatic function, it becomes suitable for Applications 1 and 4 (interaction studies and system reconstitution). If inactive, its use should be restricted to Application 2 (antibody development). Application 2 can proceed immediately regardless of functional status. This systematic approach ensures reliable interpretation of results and appropriate resource allocation.