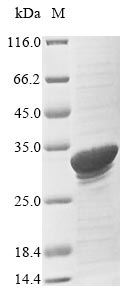
The expression of recombinant Bovine SAA1 protein includes the construction of the expression vector containing the recombinant DNA and the transformation of the expression vector into the E.coli, which provides a variety of macromolecules and components required for transcription and translation. The recombinant DNA was formed by fusing the N-terminal 6xHis-SUMO tag and C-terminal Myc tag sequence to the designated sequence encoding the 19-130aa of the Bovine SAA1 protein. This N-terminal 6xHis-tagged recombinant Bovine SAA1 protein is also characterized by high purity, >85%. Under SDS-PAGE condition, this recombinant SAA1 protein migrated to the band of about 33 kDa molecular weight.
SAA1 protein is expressed primarily in the liver and circulates in low levels in the blood. Although its function is not fully understood, serum amyloid A1 appears to play a role in the immune system. SAA1 is closely associated with high-density lipoprotein. Additionally, SAA1 interacts with a number of mammalian proteins. These receptors include the G protein-coupled chemoattractant receptor FPR2. Certain studies indicated that SAA1 might contribute to the development of atherosclerosis. SAA1 is also presented in macrophages and epithelial cells in various tissues. It has been found to enhance local Th17 response in the gut. Other studies have shown that native human SAA1 retains some of the cytokine-like activities such as the G-CSF-induction capability. In a case, the findings suggested that a mutation in the SAA1 promoter causes hereditary amyloid A amyloidosis. Besides, as SAA1 transcriptionally activated by STAT3, it could accelerate renal interstitial fibrosis by inducing endoplasmic reticulum stress; SAA1knockdown promotes the apoptosis of glioblastoma cells via downregulation of AKT signaling.