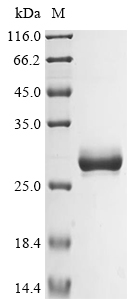
Recombinant Human Basement membrane-specific heparan sulfate proteoglycan core protein (HSPG2) is produced using a mammalian cell expression system, which appears to ensure proper folding and post-translational modifications. This product contains a partial sequence spanning amino acids 4197 to 4391. For practical purposes, it's tagged with an N-terminal 10xHis-tag and a C-terminal Myc-tag to make purification and detection more straightforward. SDS-PAGE analysis indicates the protein shows purity levels greater than 85%, which should make it suitable for various research applications.
HSPG2 represents an essential component of the extracellular matrix and is primarily found in basement membranes. It likely plays a critical role in maintaining structural integrity while supporting cell signaling processes. This proteoglycan seems to participate in various biological pathways—cell adhesion, proliferation, and differentiation among them. These characteristics have made it a significant focus in developmental biology, cell biology, and tissue engineering studies.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Human HSPG2 is a large, complex basement membrane protein that requires extensive post-translational modifications, including glycosylation and proper folding for its functional activity. The mammalian cell expression system is ideal for this protein as it can provide the necessary eukaryotic folding environment and post-translational modifications. However, this recombinant protein represents only a partial fragment (4197-4391aa) of the full-length protein, which may lack the complete structural context needed for some functional activities. The dual tags are relatively small and unlikely to significantly interfere with folding. Therefore, this protein has a high probability of being correctly folded, though its functional activity may be limited to domain-specific interactions rather than full HSPG2 function.
1. Antibody Development and Validation Studies
This recombinant HSPG2 fragment serves as an excellent immunogen for generating antibodies specific to the C-terminal region of human Perlecan. The mammalian expression ensures proper folding and authentic epitope presentation. The dual tags facilitate purification and provide additional epitopes for screening. Antibodies will likely recognize the native protein in biological samples.
2. Protein-Protein Interaction Studies Using Tag-Assisted Pull-Down Assays
The mammalian expression system supports proper folding for authentic protein-protein interactions specific to this domain. The dual tags provide flexibility in immobilization and detection strategies. Protein folding needs to be verified before the interaction studies. If properly folded, this protein is well-suited for studying interactions involving the C-terminal domain of HSPG2.
3. ELISA-Based Binding and Competition Assays
Mammalian expression may provide a properly folded protein reliable for immunoassay development and binding studies. Protein folding needs to be verified before experiments. If properly folded, this protein is ideal for developing immunoassays to detect HSPG2 or antibodies against it. The dual tags enable robust sandwich ELISA development for quantitative measurements.
4. Biochemical Characterization and Stability Studies
This is a priority application to understand the biophysical properties of this specific HSPG2 domain. Techniques like SEC-MALS and circular dichroism can characterize folding quality and stability. These studies provide essential quality control and characterize the domain's properties in a biologically relevant context.
Final Recommendation & Action Plan
This recombinant HSPG2 fragment expressed in mammalian cells has a high probability of proper folding and is suitable for all proposed applications, with the mammalian expression system providing significant advantages for biological relevance. The recommended approach is to begin with Application 4 (Biochemical Characterization) to confirm folding quality and domain properties. Then proceed confidently with Applications 1, 2, and 3 for antibody development, interaction studies, and assay development. For functional studies beyond this domain's scope, consider full-length HSPG2, but this fragment is excellent for domain-specific investigations.