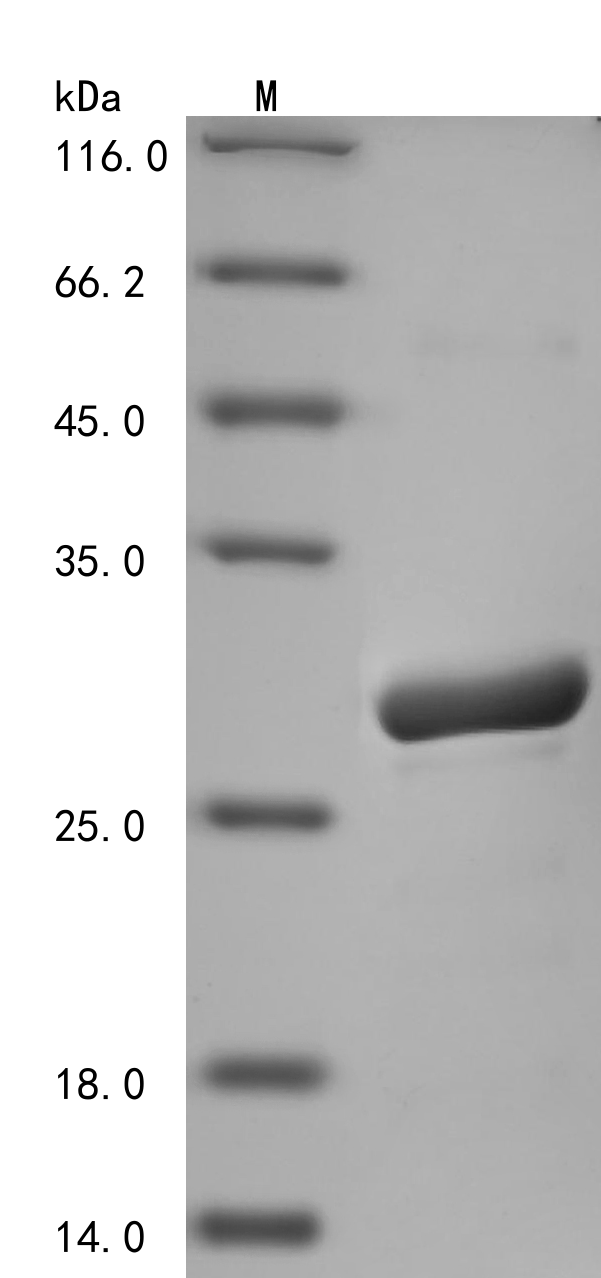
The expression region of this recombinant Human DHFR covers amino acids 1-187. The expected molecular weight for the DHFR protein is calculated to be 25.5 kDa. This DHFR protein is produced using e.coli expression system. The DHFR gene fragment has been modified by fusing the N-terminal 6xHis tag, providing convenience in detecting and purifying the recombinant DHFR protein during the following stages.
Human dihydrofolate reductase (DHFR) is a pivotal enzyme in cellular metabolism, essential for the synthesis of nucleic acids. It catalyzes the conversion of dihydrofolate to tetrahydrofolate, a vital cofactor for DNA and RNA synthesis. DHFR plays a crucial role in cell division and proliferation by ensuring an adequate supply of folate-derived cofactors for nucleotide biosynthesis. Due to its significance in cellular processes, DHFR is a target for antifolate drugs, including methotrexate, commonly used in cancer chemotherapy and autoimmune disease treatment. Understanding DHFR's function is vital for developing therapeutic strategies and elucidating folate metabolism intricacies.