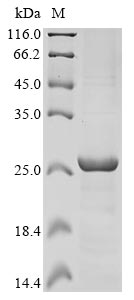
Recombinant Human Excitatory amino acid transporter 1 (SLC1A3) is produced using an E.coli expression system, with researchers focusing specifically on amino acids 146-236 of the protein. This partial protein comes with an N-terminal 6xHis-KSI tag, which appears to make purification and detection more straightforward. SDS-PAGE analysis confirms the product achieves greater than 85% purity, though this may vary between batches.
The Excitatory amino acid transporter 1 (EAAT1), which the SLC1A3 gene encodes, seems to play a critical role in moving glutamate across cell membranes. Its primary function likely involves maintaining neurotransmitter balance in the central nervous system by removing glutamate from synaptic spaces. This makes EAAT1 particularly relevant for scientists studying synaptic transmission and neural health.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Based on the provided information, the recombinant human SLC1A3 fragment is expressed in E. coli, a prokaryotic system that is fundamentally unsuitable for producing functional eukaryotic membrane transport proteins. SLC1A3 is a complex transmembrane protein with multiple membrane-spanning domains that require precise folding, proper membrane insertion, and specific oligomerization for function. The expressed fragment (146-236aa) represents only a small portion of the full-length protein and is fused to a large N-terminal 6xHis-KSI tag, which likely interferes with proper folding. E. coli lacks the eukaryotic chaperones and membrane environment necessary for the correct folding of neurotransmitter transporter domains. Since activity is unverified and the expression system is mismatched for this complex membrane protein, the protein is highly likely to be misfolded and inactive without experimental validation.
1. Antibody Development and Validation
This application is appropriate as the safest use case. The recombinant SLC1A3 fragment can serve as an immunogen for generating antibodies that recognize linear epitopes within the 146-236aa region, even if the protein is misfolded. The His-KSI tag facilitates purification and screening. However, antibodies may not recognize conformational or membrane-embedded epitopes of the full-length, properly folded SLC1A3 transporter in its native cellular context. Validation against native SLC1A3 from mammalian membranes is essential.
2. Protein-Protein Interaction Studies
This application is high-risk without folding validation. While the His-tag enables technical feasibility for pull-down assays, if the SLC1A3 fragment is misfolded (as expected in E. coli), it will not present physiological interaction interfaces. The 146-236aa region may contain partial domains that require a specific membrane context for proper folding. Identified interactions are likely to be non-physiological artifacts. This application requires prior confirmation of proper folding.
3. Epitope Mapping and Binding Studies
This application is feasible for linear epitope mapping but problematic for conformational studies. The defined fragment can help map antibody recognition to the 146-236aa region. However, if the protein is misfolded, surface plasmon resonance or binding studies will not reflect native protein-ligand interactions. This application should be limited to linear epitope characterization unless proper folding is demonstrated.
4. Biochemical Characterization and Stability Studies
This application is well-suited for assessing the recombinant product itself. Techniques like circular dichroism spectroscopy and thermal stability assays can evaluate the fragment's structural properties. However, results will characterize the E. coli-expressed product and may not reflect the behavior of this region within the full-length, membrane-embedded native protein.
Final Recommendation & Action Plan
Given the high probability of misfolding due to E. coli expression and the presence of a large fusion tag, recommend first performing biophysical characterization (circular dichroism for secondary structure analysis, size-exclusion chromatography for oligomeric state) to assess folding quality. Antibody development and linear epitope mapping can proceed as the lowest-risk applications. Avoid all protein interaction studies and conformational binding assays until proper folding is validated. For reliable SLC1A3 functional studies, obtain full-length protein expressed in mammalian or insect cell systems capable of proper membrane insertion and folding. Always include appropriate controls and validate findings with native SLC1A3 when possible.