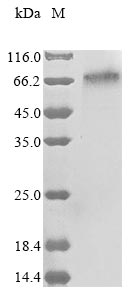
Recombinant Human Integrin beta-like protein 1 (ITGBL1) is produced using a mammalian expression system, which appears to ensure proper protein folding and post-translational modifications. The full-length mature protein spans amino acids 24 to 494. It comes with an N-terminal 10xHis tag and a C-terminal Myc tag - features that make purification and detection more straightforward. SDS-PAGE analysis shows the protein reaches greater than 85% purity, a level that seems adequate for most research applications.
ITGBL1 belongs to the integrin family, proteins known for their crucial roles in cell adhesion and signaling. Interestingly though, ITGBL1 itself doesn't bind ligands the way typical integrins do. Instead, it likely modulates cellular interactions and signaling pathways through other mechanisms. Researchers have found it relevant to studies of cell migration and tissue organization, and there may be novel roles in cellular communication yet to be discovered.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Based on the provided information, the folding state and bioactivity of this recombinant human ITGBL1 protein are unknown and cannot be assumed. Although the ITGBL1 protein is expressed in a mammalian system, which is more likely to support proper folding and post-translational modifications compared to prokaryotic systems, the presence of both N-terminal and C-terminal tags (10xHis and Myc) may still interfere with the native structure or function. The activity has not been validated, so it is uncertain whether the protein is correctly folded or bioactive. Therefore, applications that rely on specific biological activity or native conformation must be considered speculative without experimental confirmation.
1. Protein-Protein Interaction Studies Using Pull-Down Assays
The N-terminal 10xHis tag allows for immobilization on nickel-based matrices for pull-down experiments to identify potential binding partners. However, since the protein's folding and activity are unverified, any interactions detected may be non-specific or artifacts if the protein is misfolded. The dual tags provide flexibility for validation, but results should be interpreted with caution and confirmed using orthogonal methods, such as co-immunoprecipitation with endogenous protein, to ensure biological relevance.
2. Antibody Development and Validation
This recombinant ITGBL1 protein is suitable as an immunogen for generating polyclonal or monoclonal antibodies. The mammalian expression system may produce proteins with folding and modifications similar to the native form, potentially increasing the likelihood of antibodies with physiological relevance. However, antibody specificity must be rigorously validated against native ITGBL1 in human tissues or cell lines, as antibodies may primarily recognize linear epitopes or the tags rather than conformational epitopes of the native protein. The Myc tag can serve as a positive control for expression and loading.
3. ELISA-Based Quantitative Assays
The dual-tagged ITGBL1 protein can be used to develop sandwich ELISA assays for research purposes. However, the assay's ability to accurately quantify native ITGBL1 in biological samples (e.g., cell culture supernatants or tissue extracts) is uncertain without validation against a native standard. The ELISA may be reliable for detecting the recombinant ITGBL1 protein itself, but it may not correlate with endogenous ITGBL1 levels due to potential differences in folding, modification, or tag interference. Assay development should include validation with native samples to ensure specificity.
4. Biochemical Characterization and Stability Studies
This recombinant ITGBL1 protein is well-suited for biochemical characterization, including thermal stability, pH sensitivity, and buffer optimization studies. Techniques like dynamic light scattering, circular dichroism, and analytical ultracentrifugation can provide insights into the protein's biophysical properties. This application is valid as it focuses on intrinsic properties of the recombinant protein and does not require native bioactivity. However, the results may not fully represent the native ITGBL1 due to the presence of tags, so conclusions should be drawn cautiously.
Final Recommendation & Action Plan
The immediate priority is to validate the recombinant ITGBL1 protein's folding and bioactivity before proceeding with functional studies. This can be done using techniques such as circular dichroism to assess secondary structure, size-exclusion chromatography to monitor oligomerization, and functional assays (e.g., binding assays with known partners if available) to confirm activity. Once activity is verified, the protein can be reliably used for interaction studies, antibody development, and ELISA applications. If activity is not confirmed, the protein should be limited to biochemical characterization and as a tool for generating antibodies, with the understanding that they may not recognize native ITGBL1. Always include appropriate controls and validation steps in experiments to ensure data reliability.