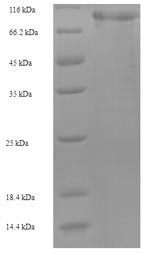
The synthesis of the recombinant plasmid containing the gene encoding the Human MASP1 protein (20-699aa) is the first step to produce the recombinant Human MASP1 protein. After that, the recombinant plasmid is transformed into e.coli cells. e.coli cells capable of enduring a specific antibiotic are selected, demonstrating successful uptake of the recombinant plasmid. The e.coli cells containing the recombinant plasmid are cultured under conditions that encourage the expression of the gene of interest. A N-terminal 6xHis-SUMO tag is linked to the protein. Following expression, affinity purification is employed to isolate and purify the recombinant Human MASP1 protein from the cell lysate. Denaturing SDS-PAGE is applied to resolve the resulting recombinant Human MASP1 protein, indicating a purity level exceeding 90%.