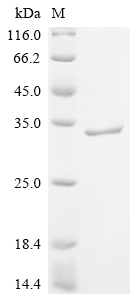
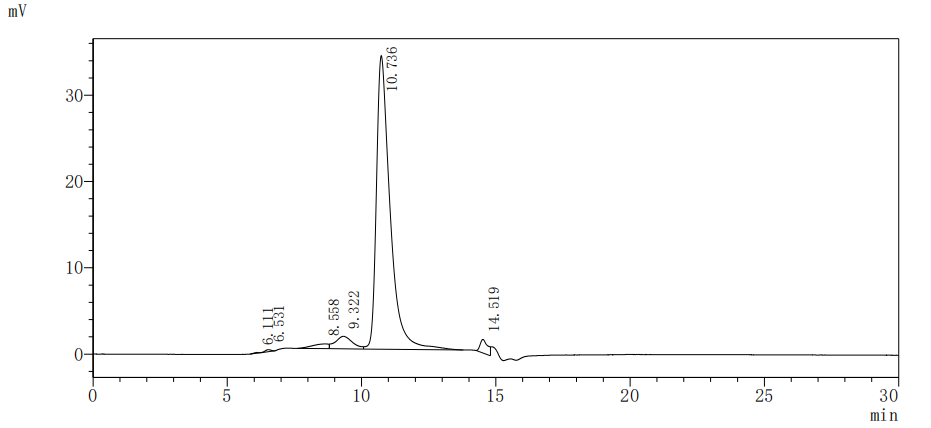
Recombinant Human Pulmonary Surfactant-Associated Protein A1 (SFTPA1) gets produced in a mammalian cell system, which appears to help with proper folding and post-translational modifications. The protein includes the full-length mature sequence from amino acids 21 to 248 and comes with a C-terminal hFc1 tag for enhanced stability and detection. This product shows purity greater than 95%, as verified by SDS-PAGE, making it suitable for various research applications.
Pulmonary surfactant-associated protein A1 seems to be a crucial component of the pulmonary surfactant system. It likely plays a significant role in the innate immune defense of the lungs. The protein gets involved in regulating surfactant homeostasis and participates in pathways that may be critical for host defense mechanisms. Respiratory researchers often focus on this protein, particularly when studying lung immunity and surfactant-related disorders.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Based on the provided information, the folding state and bioactivity of this recombinant SFTPA1 protein are unknown and cannot be assumed. While the protein is expressed in a mammalian system (which is favorable for proper folding and post-translational modifications compared to prokaryotic systems) and shows high purity by two orthogonal methods (SDS-PAGE and SEC-HPLC), the presence of a large C-terminal hFc1 tag is a significant variable. This tag could potentially sterically hinder functional domains or alter the protein's oligomerization state, which is critical for a collectin family protein like SFTPA1 that functions through its carbohydrate recognition domain (CRD). SEC-HPLC indicating >90% purity suggests a monodisperse preparation, which is a positive indicator of folding homogeneity, but does not confirm native bioactivity (e.g., binding to pathogens or surfactant lipids). Therefore, applications relying on specific biological interactions are speculative without validation.
1. Antibody Development and Validation Studies
This high-purity recombinant SFTPA1 protein is suitable as an immunogen for generating antibodies. The mammalian expression system increases the likelihood of producing antibodies that recognize epitopes similar to those on the native protein. Antibodies generated will be against the SFTPA1-hFc1 fusion protein. Their ability to recognize and functionally interact with the native, oligomeric SFTPA1 complex in lung surfactant must be empirically validated. The protein is excellent for initial antibody screening and as a reference standard for the recombinant SFTPA1 itself.
2. Protein-Protein Interaction Studies
The C-terminal hFc1 tag allows for efficient capture using Protein A/G for pull-down or co-IP experiments. However, the utility for identifying biological binding partners is entirely contingent on the protein being correctly folded. The Fc tag itself can cause non-specific interactions through Fc receptors. More critically, if the protein's CRD is occluded or misfolded, it will not bind its natural ligands (e.g., pathogens, surfactant lipids). This application should be approached with caution, and any identified interactions must be rigorously confirmed with native SFTPA1.
3. ELISA and Immunoassay Development
The hFc-tagged protein can be used to develop ELISA assays. However, its utility is context-dependent. It is well-suited for developing assays to detect antibodies raised against this recombinant SFTPA1 protein (Application 1). It is not suitable for quantitative assays for measuring SFTPA1 levels in biological samples because the recombinant SFTPA1 protein is antigenically distinct from the native, oligomeric SFTPA1 present in vivo. An assay built with this reagent would not accurately quantify the endogenous protein.
4. Biochemical Characterization and Functional Studies
This recombinant SFTPA1 protein is well-suited for detailed biochemical and biophysical characterization. Techniques like SEC-HPLC, dynamic light scattering, and thermal stability assays can provide valuable insights into the oligomerization state, stability, and solution behavior of this specific SFTPA1-hFc1 fusion protein. This application is valid as it focuses on intrinsic physical properties. However, the data characterizes the recombinant SFTPA1 protein, and findings may not be directly transferable to the native SFTPA1 protein due to the influence of the large Fc tag on oligomerization and stability.
Final Recommendation & Action Plan
The immediate recommendation is to leverage this high-quality reagent for applications it is validated for: antibody development (Application 1) and biophysical characterization of the fusion construct itself (Application 4). For Applications 2 and 3, which rely on native bioactivity, the crucial first step is to validate the protein's function. A logical initial experiment would be a binding assay using known ligands of SFTPA1, such as specific carbohydrates (e.g., mannan) or surfactant lipids. If binding activity is confirmed, the protein becomes suitable for interaction studies and could be considered for developing functional assays. If activity is not confirmed, its use should be restricted to Applications 1 and 4, and the results of interaction studies (Application 2) would be considered unreliable for understanding native SFTPA1 biology. The Fc tag is a major confounding factor for functional studies.