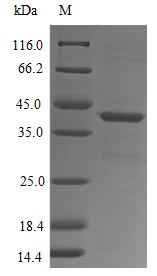
Amino acids 306-350 constitute the expression domain of recombinant Human FPR1. The expected molecular weight for the FPR1 protein is calculated to be 34.9 kDa. Expression of this FPR1 protein is conducted in e.coli. The FPR1 gene fragment has been modified by fusing the N-terminal 10xHis-GST tag and C-terminal Myc tag, providing convenience in detecting and purifying the recombinant FPR1 protein during the following stages.
The human fMet-Leu-Phe receptor (FPR1) is a GPCR primarily expressed on immune cells such as neutrophils and macrophages. FPR1 is crucial in mediating chemotaxis, phagocytosis, and inflammatory responses. It recognizes N-formylated peptides, which are often released by bacteria during infection. Upon ligand binding, FPR1 activates downstream signaling cascades, leading to cytoskeletal rearrangements, immune cell recruitment, and antimicrobial activities. FPR1's involvement in immune responses makes it a potential target for therapeutic interventions in infectious diseases and inflammatory disorders. Understanding its function provides insights into the regulation of immune cell behavior and offers avenues for developing novel immunomodulatory strategies.