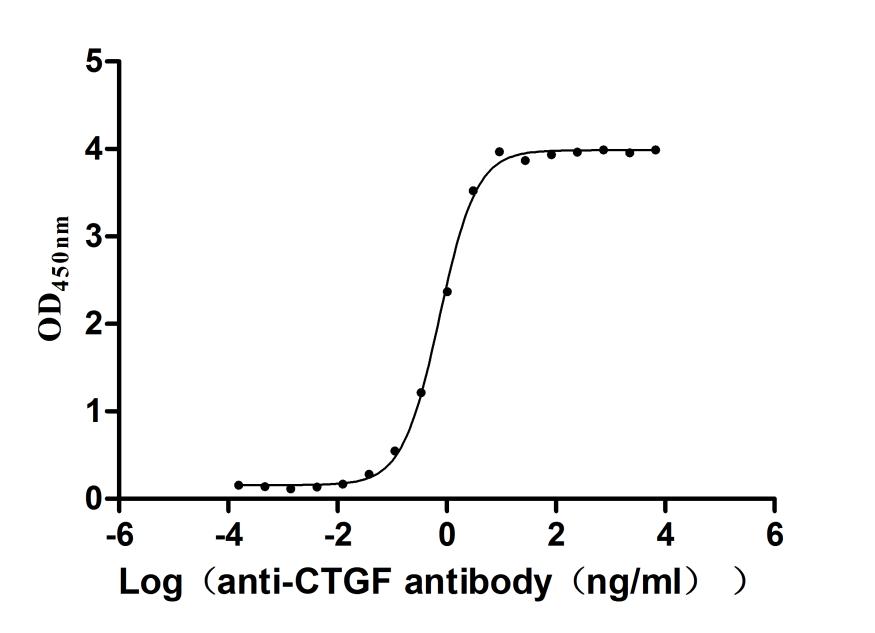
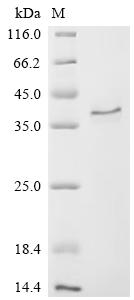
The recombinant Macaca mulatta CCN2 protein is an active, high-purity protein prepared in mammalian cells to preserve its native conformation and biological function. It comprises amino acids 27 to 349 of the full-length mature CCN2 sequence and is engineered with a C-terminal 10xHis tag for ease of purification and detection. Provided as a lyophilized powder, this recombinant CCN2 protein exceeds 95% purity as verified by SDS-PAGE. Functional validation through ELISA confirms its specific binding to the anti-CTGF recombinant antibody (CSB-RA006147MA2HU) when immobilized at 1 μg/mL, with an EC50 between 0.6886 and 0.7825 ng/mL. These attributes make it well-suited for applications in fibrosis research, growth factor signaling studies, and antibody validation.
The CCN2 protein, also known as connective tissue growth factor (CTGF), plays a significant role in various biological processes, including cell proliferation, differentiation, and ECM (extracellular matrix) production. In primates, including Macaca mulatta, the understanding of CCN2 is critical for elucidating its physiological and pathological implications in both human and veterinary medicine.
CCN2 is known to be involved in tissue development and repair. It has been identified as a key factor linking mechanical stimuli with cellular responses, influencing wound healing and fibrogenesis. In tissues of Macaca mulatta, CCN2 expression has been associated with pathological conditions such as fibrosis and has shown significant variation in expression levels depending on tissue type and physiological state. Studies have highlighted that alterations in CCN2 expression correlate with other markers of fibrosis, thereby emphasizing its role in fibrotic diseases [1][2][3]. Specifically, CCN2 can modulate the behavior of various cell types, including fibroblasts and endothelial cells, which are critical for maintaining tissue integrity and reparative processes [4][5][3].
In the context of rhesus macaques, CCN2 has been identified as a potential biomarker for various health conditions, including chronic inflammatory diseases and cancer. Research indicates that higher levels of CCN2 may be indicative of an increased fibrotic response, suggesting its utility in both diagnostic and therapeutic contexts [6][7][8]. Furthermore, studies have demonstrated that CCN2 can influence immune responses, potentially impacting the pathogenesis of autoimmune disorders prevalent in certain primate models [9][10].
Moreover, the comparative analysis of CCN2 in Macaca mulatta alongside other nonhuman primates underscores the evolutionary conservation of this protein and its functional importance across species. This conservation facilitates the use of these animals in preclinical models for human diseases, allowing for more effective translational research outcomes [11][12][13]. The incorporation of CCN2 studies into the broader genomic and proteomic frameworks available for Macaca mulatta enhances the understanding of its function and regulation within the cellular milieu, providing insights into both fundamental biology and potential clinical applications [14][15][16].
References:
[1] M. Pomrenze, T. Fetterly, D. Winder, & R. Messing. The corticotropin releasing factor receptor 1 in alcohol use disorder: still a valid drug target? Alcoholism Clinical and Experimental Research, vol. 41, no. 12, p. 1986-1999, 2017. https://doi.org/10.1111/acer.13507
[2] Z. Waibler, L. Sender, et al. Signaling signatures and functional properties of anti-human cd28 superagonistic antibodies. Plos One, vol. 3, no. 3, p. e1708, 2008. https://doi.org/10.1371/journal.pone.0001708
[3] S. Handley, C. Desai, et al. Siv infection-mediated changes in gastrointestinal bacterial microbiome and virome are associated with immunodeficiency and prevented by vaccination. Cell Host & Microbe, vol. 19, no. 3, p. 323-335, 2016. https://doi.org/10.1016/j.chom.2016.02.010
[4] Z. Su, J. Zhang, et al. Species specific exome probes reveal new insights in positively selected genes in nonhuman primates. Scientific Reports, vol. 6, no. 1, 2016. https://doi.org/10.1038/srep33876
[5] N. Galtier. An approximate likelihood method reveals ancient gene flow between human, chimpanzee and gorilla. 2023. https://doi.org/10.1101/2023.07.06.547897
[6] E. Rathke and J. Fischer. Social aging in male and female barbary macaques. American Journal of Primatology, vol. 83, no. 11, 2021. https://doi.org/10.1002/ajp.23272
[7] H. Harvala, C. Sharp, E. Ngole, É. Delaporte, M. Peeters, & P. Simmonds. Detection and genetic characterization of enteroviruses circulating among wild populations of chimpanzees in cameroon: relationship with human and simian enteroviruses. Journal of Virology, vol. 85, no. 9, p. 4480-4486, 2011. https://doi.org/10.1128/jvi.02285-10
[8] E. Robertson, S. Boehnke, et al. Comparison of cerebrospinal fluid biomarkers relevant to neurodegenerative diseases in healthy cynomolgus and rhesus macaque monkeys. 2021. https://doi.org/10.1101/2021.03.01.433384
[9] A. Zimin, A. Cornish, et al. A new rhesus macaque assembly and annotation for next-generation sequencing analyses. Biology Direct, vol. 9, no. 1, 2014. https://doi.org/10.1186/1745-6150-9-20
[10] J. Mallick. Mammals of kalimpong hills, darjeeling district, west bengal, india. Journal of Threatened Taxa, vol. 4, no. 12, p. 3103-3136, 2012. https://doi.org/10.11609/jott.o2418.3103-36
[11] P. Najarro, H. Lee, J. Fox, J. Pease, & G. Smith. Yaba-like disease virus protein 7l is a cell-surface receptor for chemokine ccl1. Journal of General Virology, vol. 84, no. 12, p. 3325-3336, 2003. https://doi.org/10.1099/vir.0.19591-0
[12] L. Fu, X. Li, W. Zhang, et al. A comprehensive profiling of t- and b-lymphocyte receptor repertoires from a chinese-origin rhesus macaque by high-throughput sequencing. Plos One, vol. 12, no. 8, p. e0182733, 2017. https://doi.org/10.1371/journal.pone.0182733
[13] A. Tanaka and S. Watanabe. Can cytoplasmic donation rescue aged oocytes?. Reproductive Medicine and Biology, vol. 18, no. 2, p. 128-139, 2018. https://doi.org/10.1002/rmb2.12252
[14] J. Almeida, A. Pinho, J. Oliveira, O. Fajarda, & D. Pratas. Gto: a toolkit to unify pipelines in genomic and proteomic research. Softwarex, vol. 12, p. 100535, 2020. https://doi.org/10.1016/j.softx.2020.100535
[15] J. Satkoski, R. Malhi, S. Kanthaswamy, R. Tito, V. Malladi, & D. Smith. Pyrosequencing as a method for snp identification in the rhesus macaque (macaca mulatta). BMC Genomics, vol. 9, no. 1, p. 256, 2008. https://doi.org/10.1186/1471-2164-9-256
[16] W. Nix, B. Jiang, K. Maher, E. Strobert, & M. Oberste. Identification of enteroviruses in naturally infected captive primates. Journal of Clinical Microbiology, vol. 46, no. 9, p. 2874-2878, 2008. https://doi.org/10.1128/jcm.00074-08