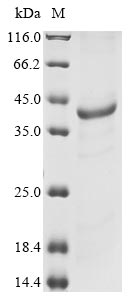
Recombinant Mouse Interferon-induced helicase C domain-containing protein 1 (Ifih1) is expressed in E. coli, representing the 700-1025 amino acid region of the protein. This partial protein carries an N-terminal 6xHis tag, which makes purification and detection more straightforward. The product reaches a purity level greater than 85% as determined by SDS-PAGE, ensuring high-quality results for research applications.
Interferon-induced helicase C domain-containing protein 1 (Ifih1) appears to be a crucial component of the innate immune response. It acts as a pattern recognition receptor, detecting viral RNA within cells and triggering antiviral pathways. Ifih1 likely plays a significant role in the body's defense mechanisms, making it an important subject of study in immunology and virology research.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Based on the provided information, the recombinant Ifih1 C-terminal domain fragment is unlikely to be correctly folded or bioactive without experimental validation. Ifih1 is a large, multi-domain protein that requires precise folding and potential post-translational modifications for its function as a viral RNA sensor. The E. coli expression system lacks the eukaryotic folding machinery and may not support proper folding of this complex eukaryotic protein domain. The partial nature of the protein (700-1025aa) represents only the C-terminal region, which may lack critical interactions with other domains necessary for correct folding. While the 6xHis tag is relatively small, it could still cause steric hindrance. The purity >85% indicates some impurities that may include misfolded species. Without validation through biophysical methods and functional assays, the protein's structural integrity and functionality cannot be assumed.
1. Protein-Protein Interaction Studies
This recombinant Ifih1 fragment can be used for interaction studies only if proper folding is confirmed. The His-tag enables immobilization but may cause non-specific binding or steric interference. If misfolded, identified interactions may be artifactual. Validate folding through circular dichroism or size-exclusion chromatography before use, and include appropriate controls (e.g., tag-only protein) to ensure specificity.
2. Antibody Development and Validation
The protein can generate antibodies, but if misfolded, they may not recognize native Ifih1 epitopes. The antibodies will primarily target linear epitopes of the C-terminal region rather than conformational epitopes. Validate resulting antibodies against full-length, properly folded Ifih1 to ensure biological relevance. The high purity supports immunization, but doesn't guarantee functional antibodies.
3. Structural and Biochemical Characterization
This fragment is suitable for biophysical characterization only if folding is validated. Techniques like circular dichroism can assess secondary structure, but the His-tag may influence results. For meaningful structural insights, remove the tag and confirm the domain maintains native-like conformation. Comparative studies with full-length Ifih1 require both proteins to be properly folded.
4. In Vitro Functional Domain Analysis
Functional studies require demonstration that the fragment is properly folded and bioactive. The isolated domain may lack interactions necessary for full activity, and the E. coli expression may not support correct folding. Validate functionality through known Ifih1 assays before concluding domain-specific functions. Use a full-length protein as a positive control where possible.
Final Recommendation & Action Plan
This recombinant Ifih1 fragment requires thorough validation before reliable use. First, assess folding through biophysical methods (circular dichroism, thermal stability assays). Test functionality if possible, though the isolated domain may have limited activity. For interaction studies, include rigorous controls and confirm findings with full-length protein. For antibody production, characterize sera against native Ifih1. Consider using eukaryotic expression systems for studies requiring proper folding and post-translational modifications. Always confirm fragment folding and stability before functional applications.