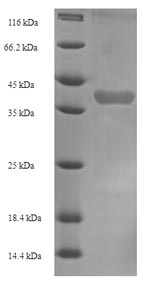
Recombinant Mouse Kallikrein 1-related peptidase b22 (Klk1b22) gets produced in an E.coli expression system and contains amino acids 25-259 of the mature protein. The protein comes with an N-terminal 6xHis-SUMO tag, which makes purification and solubility much more manageable. SDS-PAGE analysis shows it reaches over 90% purity - pretty solid for most research work.
Klk1b22 belongs to the kallikrein-related peptidase family, a group known for proteolytic activity across different physiological processes. These enzymes appear to play important roles in inflammation pathways, blood pressure regulation, and tissue remodeling. Though the functional dynamics of Klk1b22 may provide insights into these critical biological processes, much about its specific role remains to be fully understood.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
1. Antibody Development and Immunoassay Optimization
This recombinant Klk1b22 serves as an excellent immunogen for generating specific antibodies against mouse kallikrein 1-related peptidase b22. The full-length mature protein sequence (25-259aa) ensures comprehensive epitope coverage, and the high purity (>90%) minimizes antibodies against contaminants. The His-SUMO tag facilitates purification and immobilization during antibody screening and validation in ELISA, Western blot, or immunoprecipitation assays.
2. Protein-Protein Interaction Studies Using Tag-Assisted Pull-Down Assays
The E.coli expression system may not provide the post-translational modifications and the correct folding environment required for eukaryotic proteins, especially for proteases, which may require specific disulfide bond formation and conformation. SUMO tags may improve solubility, but they cannot guarantee functional folding. Therefore, the probability of this protein folding correctly and being active is relatively low and requires experimental verification. If correctly folded, the protein could identify physiological binding partners. However, a misfolded Klk1b22 may expose hydrophobic regions, leading to non-specific (false-positive) binding or failing to present genuine interaction interfaces, causing false negatives. The large SUMO tag may sterically hinder binding sites. Data interpretation would be challenging without validation using a natively folded protein.
3. Biochemical Characterization and Stability Studies
This is the essential first step to assess the protein's physical properties and folding state. Techniques like size-exclusion chromatography with multi-angle light scattering (SEC-MALS) can determine oligomeric state and homogeneity, while circular dichroism (CD) spectroscopy can analyze secondary structure content and thermal stability. These studies provide critical quality control data for evaluating the protein's suitability for functional applications.
4. Comparative Structural and Functional Analysis Within the Kallikrein Family
This protein can be used for sequence-based comparisons and immunological cross-reactivity studies with other kallikrein family members. However, comparative functional analyses (e.g., enzymatic activity or substrate specificity) would be invalid without confirmed native folding and activity. Structural comparisons via Western blotting or epitope mapping are feasible. It is useful for non-functional comparative studies, but functional analyses require a properly folded and active enzyme.
Final Recommendation & Action Plan
The recombinant Klk1b22 is reliable for antibody development and biochemical characterization but requires rigorous validation for functional studies due to uncertainties in folding and activity. The immediate priority is Application 3 (Biochemical Characterization) to assess protein folding, stability, and oligomeric state through SEC-MALS and CD spectroscopy. If the protein demonstrates monodisperse behavior and structured spectra, proceed to validate enzymatic activity using specific substrates. Once activity is confirmed, Applications 2 and 4 (functional aspects) can be considered. Application 1 (Antibody Development) can proceed immediately regardless of folding status. Without activity validation, limit use to non-functional applications to avoid misleading results. This systematic approach ensures reliable data interpretation.