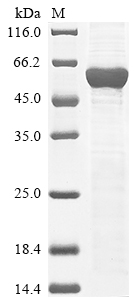
Recombinant Mouse Leucine-rich repeat LGI family member 3 (Lgi3) is produced in E. coli and consists of the full length of the mature protein, covering amino acids 31-548. The protein carries an N-terminal 6xHis tag, which makes purification and detection more straightforward. SDS-PAGE analysis shows the product achieves greater than 85% purity—a level that appears adequate for most research applications.
Lgi3 belongs to the leucine-rich repeat LGI family, which seems to be involved in various cellular processes. This protein may play roles in signal transduction pathways and has drawn interest in studies examining cellular communication and neuronal function. Its apparent importance in modulating cell-to-cell interactions makes it a potentially valuable target for research in neurobiology and cell biology.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Mouse Lgi3 is a leucine-rich repeat protein that likely requires proper folding for its function in protein-protein interactions and neuronal signaling. The E. coli expression system can produce soluble proteins, but it may not fully replicate the eukaryotic folding environment, potentially affecting disulfide bond formation or tertiary structure. The N-terminal 6xHis tag is small and unlikely to significantly interfere with folding. While the protein may be soluble and partially folded, the probability of correct folding with full functional activity is moderate but requires experimental validation. The high purity (>85%) supports reliable use in various assays.
1. Protein-Protein Interaction Studies Using His-Tag Pull-Down Assays
Protein-protein interactions depend on precise tertiary structure. While E. coli may produce folded protein, validation is needed to ensure native conformation. If correctly folded, the His-tag allows for effective pull-down assays to identify binding partners involved in Lgi3's signaling pathways. However, misfolding may lead to non-specific binding or false negatives. The leucine-rich repeat domains are interaction-prone, but results should be confirmed with complementary methods.
2. Antibody Development and Validation
This recombinant Lgi3 serves as an excellent immunogen for generating antibodies against mouse Lgi3. The full-length mature sequence ensures comprehensive epitope coverage. The His-tag facilitates purification and screening. Antibodies will be valuable for detecting Lgi3 in Western blot, ELISA, or immunohistochemistry.
3. Biochemical Characterization and Stability Studies
This is a priority application to assess the protein's physical properties. Techniques like size-exclusion chromatography, circular dichroism, and thermal shift assays can determine oligomeric state, secondary structure, and stability. These studies provide critical quality control data about the protein itself, not the native Lgi3.
4. Comparative Species Analysis in Cross-Reactivity Studies
This protein is well-suited for comparative studies with Lgi3 orthologs from other species, focusing on sequence-based comparisons or immunological cross-reactivity. However, functional comparisons (e.g., binding affinity) require validated folding and activity.
Final Recommendation & Action Plan
This recombinant Lgi3 is reliable for antibody development and biochemical characterization but requires folding validation before use in interaction studies. The immediate priority is Application 3 (Biochemical Characterization) to assess folding quality via biophysical methods. If folding is confirmed, proceed with Application 1 (Interaction Studies) with caution and validation. Applications 2 and 4 (Antibody Development and Comparative Analysis) can proceed immediately for non-functional uses. For functional studies, consider using eukaryotic expression systems if folding is inadequate. This approach ensures appropriate application based on protein quality.