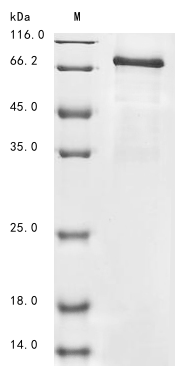
Recombinant Mouse Procollagen-lysine,2-oxoglutarate 5-dioxygenase 2 (Plod2) gets expressed in a baculovirus system. The protein covers the full-length mature sequence from amino acids 26-737. A C-terminal 6xHis-Myc tag is attached to make purification and detection more straightforward. SDS-PAGE analysis shows the purity exceeds 85%, which appears to provide reliable performance for research work.
Plod2 functions as an enzyme that's crucial for collagen biosynthesis. Its main job involves hydroxylating lysine residues in collagen-like peptides. This modification seems essential for maintaining collagen fiber stability and function, which directly affects connective tissue integrity. Research interest in Plod2 likely stems from its role in extracellular matrix formation and maintenance pathways.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Mouse Plod2 is a complex enzyme that requires precise folding, proper active site formation, and specific cofactor binding (Fe²⁺, 2-oxoglutarate, ascorbate) for its dioxygenase activity in collagen cross-linking. The baculovirus-insect cell expression system provides a eukaryotic environment that supports proper protein folding and some post-translational modifications. However, Plod2's enzymatic activity depends on the correct coordination of multiple domains and cofactors. The C-terminal 6xHis-Myc tag may interfere with the protein's C-terminal structural organization or functional domains. While the expression system increases folding probability, experimental validation is essential to confirm structural integrity and enzymatic activity.
1. Enzyme Kinetics and Substrate Specificity Studies
This application requires rigorous functional validation. Plod2's enzymatic activity depends on precise active site formation and cofactor binding. If correctly folded and active (verified), the protein is suitable for kinetic studies. If misfolded/inactive (unverified), kinetic measurements will yield biologically meaningless results. The C-terminal tag may potentially interfere with substrate access or allosteric regulation.
2. Protein-Protein Interaction Mapping
This application carries a significant risk without proper folding validation. Plod2 interactions with collagen substrates and regulatory proteins require precise tertiary structure. If correctly folded (verified), limited interaction studies may be possible. If misfolded/unverified, there is a high risk of non-specific binding or failure to identify genuine physiological interactions.
3. Antibody Development and Validation
This application is highly suitable regardless of folding status. Antibody development relies on antigenic sequence recognition rather than functional enzymatic activity. The full-length protein provides comprehensive epitope coverage for generating specific antibodies against Plod2.
4. Structural and Biophysical Characterization
These studies are essential priority applications for determining folding status. Comprehensive analysis should include size-exclusion chromatography to assess oligomeric state, circular dichroism spectroscopy to evaluate secondary structure, and thermal shift assays to determine stability.
5. Inhibitor Screening and Drug Discovery Research
This application carries a high risk without functional validation. Inhibitor screening requires native enzyme conformation and activity. If correctly folded and active (verified), limited screening may be possible. If misfolded/inactive (unverified), screening results will be unreliable for drug discovery applications.
Final Recommendation & Action Plan
The baculovirus expression system provides favorable eukaryotic folding conditions for this complex enzyme, but the C-terminal tag configuration and enzymatic complexity necessitate rigorous validation before functional applications. Begin with Application 4 (Structural Characterization) to assess folding quality through SEC, CD spectroscopy, and validate enzymatic activity using known Plod2 substrates and cofactors. Once correct folding and functional activity are verified, proceed cautiously with Applications 1, 2, and 5 for kinetic studies, interaction mapping, and inhibitor screening. Application 3 (antibody development) can proceed immediately. Always include appropriate controls: validate enzymatic activity with known substrates, test cofactor requirements, and consider using tag-free constructs for critical functional studies to minimize potential tag interference.