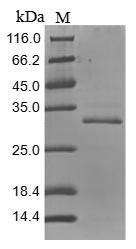
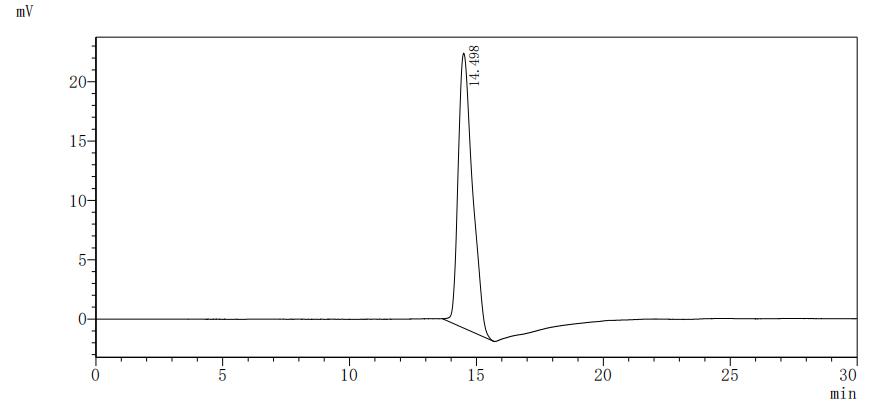
This recombinant Mouse Tenascin (Tnc) comes from a mammalian cell expression system and represents a partial protein spanning amino acids 1884-2099. The protein carries both an N-terminal 10xHis-tag and a C-terminal Myc-tag, which should make purification and detection more straightforward. SDS-PAGE analysis indicates the purity exceeds 85%, suggesting it's well-suited for laboratory work. This product is for research use only.
Tenascin appears to be a key extracellular matrix protein with important roles in how tissues develop and repair themselves. The protein seems to influence cell signaling pathways that control how cells stick together, move around, and differentiate into specialized types. Developmental biologists and cancer researchers pay particular attention to tenascin since its expression patterns often change dramatically in these contexts. Studying how this protein works and what it interacts with might reveal important details about these biological processes.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Mouse Tnc is a large extracellular matrix glycoprotein that requires precise folding, proper domain organization, and specific tertiary structure for its functional activity in cell adhesion and migration. The mammalian cell expression system provides the eukaryotic folding environment necessary for correct disulfide bond formation and potential glycosylation. However, the partial fragment (1884-2099aa, 216aa) represents only a small portion of the full-length protein (∼2200aa) and lacks the complete structural context. The dual N-terminal 10xHis-tag and C-terminal Myc-tag may cause steric interference with the protein's functional domains. While mammalian expression favors proper folding, the probability of correct folding with functional bioactivity requires experimental validation due to the truncated nature.
1. Antibody Development and Validation Studies
This application is highly suitable as antibody development relies on antigenic sequence recognition. The mammalian-expressed fragment provides proper folding and relevant post-translational modifications within this specific region. However, antibodies may not recognize conformational epitopes of the full-length, properly assembled tenascin protein in its native extracellular matrix context.
2. Protein-Protein Interaction Studies
This application carries a significant risk without functional validation. Tenascin interactions with integrins and other ECM components require precise tertiary structure and proper domain conformation. If correctly folded (verified), the fragment may identify physiological interaction partners. If misfolded/unverified, there is a high risk of non-specific binding or failure to replicate genuine extracellular matrix interactions.
3. Biochemical Characterization and Structural Studies
These studies are essential for determining folding status. Techniques should include circular dichroism spectroscopy to assess secondary structure, size-exclusion chromatography to evaluate oligomeric state, and thermal stability assays. However, results will describe this specific fragment rather than the full-length tenascin protein.
4. Cell Adhesion and Migration Assays
This application carries high risk without functional validation. Tenascin's cell adhesion properties require specific integrin-binding domains and proper tertiary structure. If correctly folded and active (verified), limited adhesion studies may be possible. If misfolded/inactive (unverified), cell behavior data will be biologically misleading.
Final Recommendation & Action Plan
The mammalian-expressed Tnc fragment with dual tags may be properly folded due to the appropriate expression system, but its functional applications are severely limited by the small size (216aa represents only ∼10% of the full-length protein) and lack of complete structural context. Begin with Application 3 (Biochemical Characterization) to validate folding quality through CD spectroscopy and SEC. Application 1 (antibody development) can proceed immediately. Applications 2 and 4 require rigorous functional validation using cell adhesion and binding assays before consideration. For reliable tenascin research, use larger domain constructs or full-length protein expressed in mammalian systems.