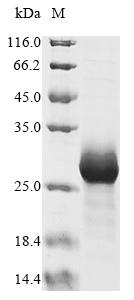
Recombinant Oryza sativa subsp. japonica Ubiquitin-conjugating enzyme E2 5A (UBC5A) is produced in E. coli and contains the full-length protein with an expression region spanning 1-147 amino acids. The protein carries an N-terminal 10xHis tag and a C-terminal Myc tag, which should make purification and detection more straightforward. SDS-PAGE analysis indicates the product achieves greater than 85% purity, suggesting it may be suitable for various research applications.
Ubiquitin-conjugating enzyme E2 5A appears to play a critical role in the ubiquitination pathway. Here, it likely functions as a key mediator that transfers ubiquitin from E1 to E3 ligases. This process seems essential for protein degradation, signal transduction, and regulation of various cellular processes. UBC5A has drawn particular interest from researchers studying proteostasis and cellular stress responses.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Ubiquitin-conjugating enzymes (E2 enzymes) require precise folding to form the catalytic core structure that interacts with E1 and E3 enzymes, and facilitates ubiquitin transfer. While E. coli can successfully express some eukaryotic proteins, the complex folding requirements and potential need for proper disulfide bond formation in E2 enzymes cannot be guaranteed without validation. The dual tagging system, particularly the large 10xHis tag at the N-terminus, may interfere with the protein's active site or functional domains. No validation data (e.g., ubiquitin conjugation assays, circular dichroism) are provided. Therefore, the protein's folding status and bioactivity remain unverified.
1. In Vitro Ubiquitin Conjugation Assays
If the recombinant UBC5A is correctly folded and functional, it could be used to study the enzymatic activity of plant E2 ubiquitin-conjugating enzymes in controlled systems, as proper folding is essential for catalytic activity in ubiquitin transfer. However, if misfolded or inactive, such assays would yield invalid results regarding ubiquitin conjugation efficiency and specificity, potentially leading to incorrect conclusions about plant ubiquitination pathways. The dual tags may also sterically hinder proper interactions with E1/E3 enzymes, even if the protein is correctly folded.
2. Protein-Protein Interaction Studies
If properly folded, the dual-tagged UBC5A could be used in pull-down assays to identify interaction partners such as E1 activating enzymes and E3 ligases, as native conformation is necessary for biologically relevant protein interactions. However, if misfolded, there is a high risk of non-specific binding or failure to recognize true biological partners, and the tags themselves might cause artificial interactions that compromise the validity of identified networks.
3. Comparative Plant E2 Enzyme Analysis
If correctly folded and active, rice UBC5A could serve as a reference for comparative studies across plant species to analyze structural and functional differences in E2 enzymes, as valid comparisons require native enzyme activity and structure. However, if misfolded, any comparative data would be biologically irrelevant and misleading for understanding species-specific adaptations in ubiquitin conjugation systems.
4. Antibody Development and Validation
This recombinant UBC5A can be used as an immunogen for antibody generation regardless of folding status, as antibodies may recognize linear epitopes even in misfolded proteins. The dual tags provide additional epitopes for detection and quantification. However, if misfolded, generated antibodies might not recognize the native, properly folded enzyme in biological contexts, limiting their utility for functional studies of endogenous UBC5A.
5. Structural and Biophysical Characterization
If properly folded, the recombinant protein could be suitable for structural studies to understand the three-dimensional architecture of rice UBC5A, as these techniques rely on native conformation for meaningful insights. However, if misfolded, structural data would misrepresent the native protein's architecture, and the presence of large tags might interfere with crystallization or NMR analysis, leading to incorrect conclusions about E2 enzyme function.
Final Recommendation & Action Plan
Before employing this recombinant UBC5A in any application, it is essential to validate its folding and bioactivity through functional ubiquitin conjugation assays with appropriate E1 and E3 components, along with biophysical characterization such as circular dichroism spectroscopy to confirm secondary structure. If validation confirms proper folding and function, proceed with applications while being cautious of potential tag interference, but if the protein is misfolded or inactive, consider using alternative expression systems (e.g., yeast or insect cells) that may better support proper folding, or obtain a commercially validated standard to ensure reliable results in all proposed applications.