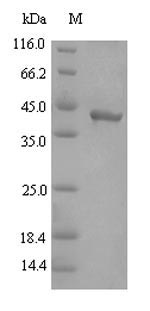
Gingipain R2 (RgpB) is a cysteine proteinase found in Porphyromonas gingivalis, a bacterium associated with periodontal disease. RgpB is one of the three types of gingipains produced by P. gingivalis, with the other two being lysine-specific gingipain (Kgp) and arginine-specific gingipain A (RgpA) [1]. RgpB is characterized by its specificity for cleaving proteins after arginine residues [2]. It is a 50 kDa proteinase that lacks the hemagglutinin/adhesin domains found in other gingipains [3]. The purification of RgpB follows similar methods used for other gingipains like HRgpA and Kgp [4].
RgpB, along with RgpA, works synergistically to release bradykinin directly from high molecular weight kininogen, mimicking the action of kallikrein [5]. Additionally, RgpB has been shown to induce neuropeptide release from dental pulp cells via PAR-2 signaling [6]. The gingipain family, which includes RgpB, plays a crucial role in the pathogenesis of periodontal disease [5]. Furthermore, RgpB has been studied for its role in activating blood coagulation factor IX [7].
References:
[1] N. Li and C. Collyer, "Gingipains fromporphyromonas gingivalis— complex domain structures confer diverse functions", European Journal of Microbiology and Immunology, vol. 1, no. 1, p. 41-58, 2011. https://doi.org/10.1556/eujmi.1.2011.1.7
[2] F. Gibson and C. Genco, "Prevention ofporphyromonas gingivalis-induced oral bone loss following immunization with gingipain r1", Infection and Immunity, vol. 69, no. 12, p. 7959-7963, 2001. https://doi.org/10.1128/iai.69.12.7959-7963.2001
[3] N. Ally, J. Whisstock, M. Sieprawska-Lupa, J. Potempa, B. Bonniec, J. Traviset al., "Characterization of the specificity of arginine-specific gingipains from porphyromonas gingivalis reveals active site differences between different forms of the enzymes", Biochemistry, vol. 42, no. 40, p. 11693-11700, 2003. https://doi.org/10.1021/bi0349726
[4] J. Potempa, J. Mikolajczyk-Pawlinska, D. Brassell, D. Nelson, I. Thøgersen, J. Enghildet al., "Comparative properties of two cysteine proteinases (gingipains r), the products of two related but individual genes ofporphyromonas gingivalis", Journal of Biological Chemistry, vol. 273, no. 34, p. 21648-21657, 1998. https://doi.org/10.1074/jbc.273.34.21648
[5] T. Imamura, "The role of gingipains in the pathogenesis of periodontal disease", Journal of Periodontology, vol. 74, no. 1, p. 111-118, 2003. https://doi.org/10.1902/jop.2003.74.1.111
[6] A. Uehara, M. Naito, T. Imamura, J. Potempa, J. Travis, K. Nakayamaet al., "Dual regulation of interleukin-8 production in human oral epithelial cells upon stimulation with gingipains from porphyromonas gingivalis", Journal of Medical Microbiology, vol. 57, no. 4, p. 500-507, 2008. https://doi.org/10.1099/jmm.0.47679-0
[7] T. Imamura, S. Tanase, T. Hamamoto, J. Potempa, & J. Travis, "Activation of blood coagulation factor ix by gingipains r, arginine-specific cysteine proteinases from porphyromonas gingivalis", Biochemical Journal, vol. 353, no. 2, p. 325, 2001. https://doi.org/10.1042/0264-6021:3530325