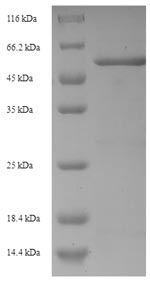
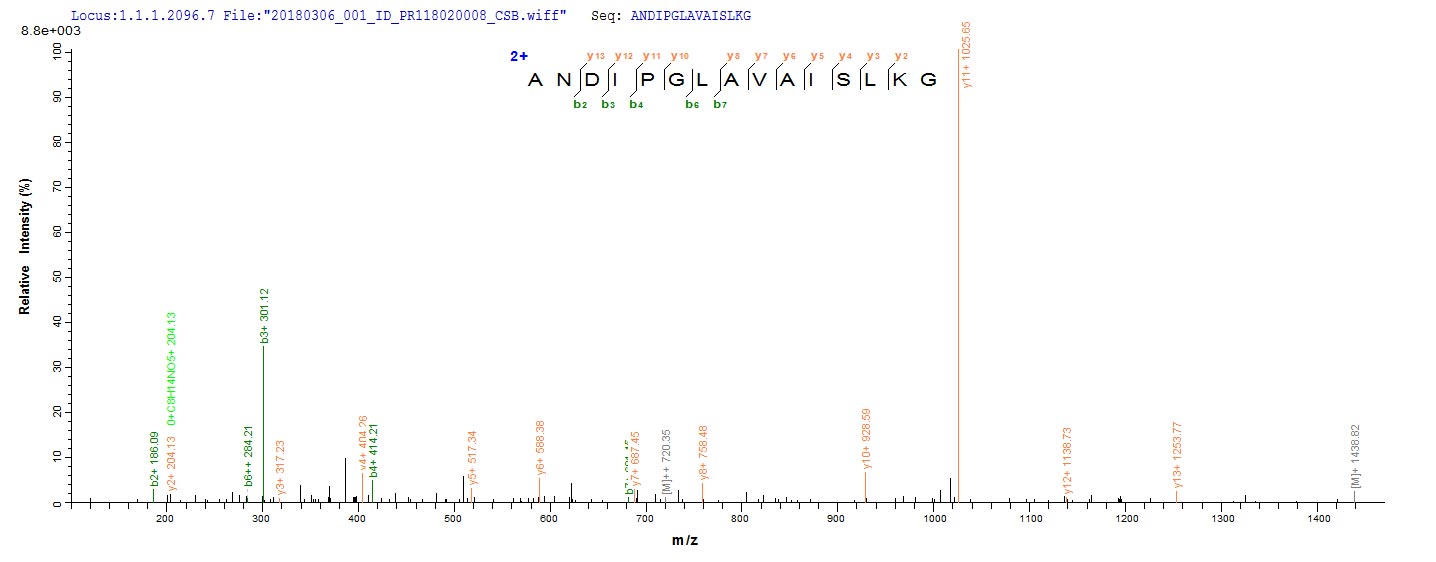
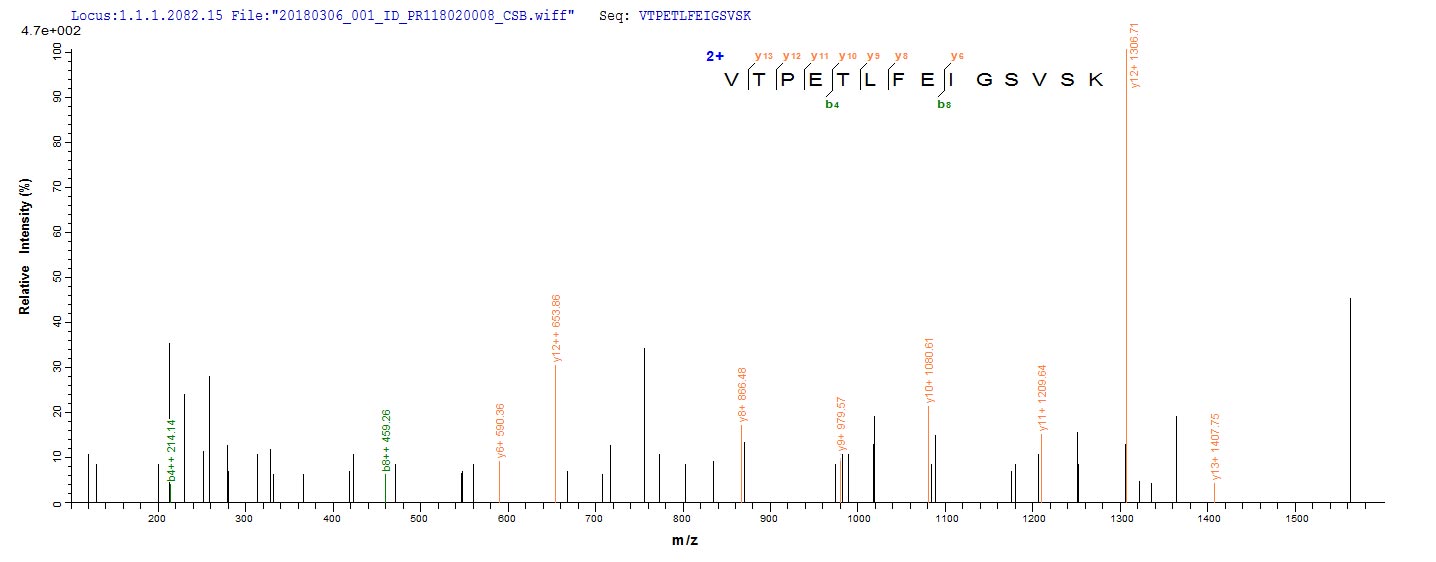
The protein AmpC is a β-lactamase enzyme that confers resistance to β-lactam antibiotics in various bacteria, including Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa [1]. AmpC is regulated by several proteins, including AmpR, a transcriptional regulator, and AmpG, a transmembrane permease that functions as a specific permease for 1,6-anhydromurapeptides, which are thought to be the signal molecules involved in ampC induction [2][3][4][5]. Additionally, AmpC has been suggested to function as a putative D,D-endopeptidase, and its overproduction is associated with β-lactam resistance [6]. The regulation of AmpC production requires three proteins: AmpG, AmpD, a cytosolic peptidoglycan-recycling amidase, and AmpR [7]. Furthermore, AmpC has a weak polybasic property and carries numerous positive charges due to the protonation of the amino groups in its backbone [8]. Penicillin-binding proteins (PBPs) also play a role in ampC regulation [9]. AmpR is a positive transcriptional regulator belonging to the LysR family of regulatory proteins [10]. The structural gene for AmpC, ampC, is located close to other genes on the chromosome, such as frdA, the structural gene for the larger subunit of fumarate reductase [11].
References:
[1] Y. Li, G. Li, L. Duo, W. Wang, C. Wang, H. Zhanget al., "Dha-1 plasmid-mediated ampc β-lactamase expression and regulation of klebsiella pnuemoniae isolates", Molecular Medicine Reports, vol. 11, no. 4, p. 3069-3077, 2014. https://doi.org/10.3892/mmr.2014.3054
[2] Y. Tsutsumi, H. Tomita, & K. Tanimoto, "Identification of novel genes responsible for overexpression of ampc in pseudomonas aeruginosa pao1", Antimicrobial Agents and Chemotherapy, vol. 57, no. 12, p. 5987-5993, 2013. https://doi.org/10.1128/aac.01291-13
[3] C. Juan, M. Macià, O. Gutiérrez, C. Vidal, J. Pérez, & A. Oliver, "Molecular mechanisms of β-lactam resistance mediated by ampc hyperproduction inpseudomonas aeruginosaclinical strains", Antimicrobial Agents and Chemotherapy, vol. 49, no. 11, p. 4733-4738, 2005. https://doi.org/10.1128/aac.49.11.4733-4738.2005
[4] C. Juan, B. Moyá, & J. Pérez, "Stepwise upregulation of thepseudomonas aeruginosachromosomal cephalosporinase conferring high-level β-lactam resistance involves three ampd homologues", Antimicrobial Agents and Chemotherapy, vol. 50, no. 5, p. 1780-1787, 2006. https://doi.org/10.1128/aac.50.5.1780-1787.2006
[5] T. Langaee, L. Gagnon, & A. Huletsky, "Inactivation of the ampd gene in pseudomonas aeruginosa leads to moderate-basal-level and hyperinducible ampc β-lactamase expression", Antimicrobial Agents and Chemotherapy, vol. 44, no. 3, p. 583-589, 2000. https://doi.org/10.1128/aac.44.3.583-589.2000
[6] R. Bishop and J. Weiner, "Coordinate regulation of murein peptidase activity and ampc β‐lactamase synthesis in escherichia coli", Febs Letters, vol. 304, no. 2-3, p. 103-108, 1992. https://doi.org/10.1016/0014-5793(92)80598-b
[7] A. Schmidtke and N. Hanson, "Role ofampdhomologs in overproduction of ampc in clinical isolates ofpseudomonas aeruginosa", Antimicrobial Agents and Chemotherapy, vol. 52, no. 11, p. 3922-3927, 2008. https://doi.org/10.1128/aac.00341-08
[8] H. Yao, R. Xiao, T. Yin, K. Shi, H. Yao, & H. Liu, "Switchable bioelectrocatalysis of glucose oxidase immobilized into multilayers with lamellar nanoparticles of amino-functionalized magnesium phyllosilicate clay", Journal of the Electrochemical Society, vol. 168, no. 4, p. 046509, 2021. https://doi.org/10.1149/1945-7111/abf442
[9] C. Liu, C. Li, Y. Chen, H. Hao, J. Liang, R. Duanet al., "Role of low-molecular-mass penicillin-binding proteins, nagz and ampr in ampc β-lactamase regulation of yersinia enterocolitica", Frontiers in Cellular and Infection Microbiology, vol. 7, 2017. https://doi.org/10.3389/fcimb.2017.00425
[10] S. Bratu, D. Landman, J. Gupta, & J. Quale, "Role of ampd, oprf and penicillin-binding proteins in β-lactam resistance in clinical isolates of pseudomonas aeruginosa", Journal of Medical Microbiology, vol. 56, no. 6, p. 809-814, 2007. https://doi.org/10.1099/jmm.0.47019-0
[11] T. Grundström and B. Jaurin, "Overlap between ampc and frd operons on the escherichia coli chromosome.", Proceedings of the National Academy of Sciences, vol. 79, no. 4, p. 1111-1115, 1982. https://doi.org/10.1073/pnas.79.4.1111