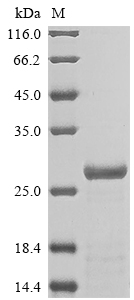
Recombinant Rat Alpha-1-acid glycoprotein (Orm1) is expressed in E. coli and includes an N-terminal 6xHis tag that makes purification more straightforward. The protein covers the complete mature sequence from amino acids 19 to 205. SDS-PAGE analysis shows the product is greater than 85% pure, which appears sufficient for most research applications. This protein is intended for research use only and not for diagnostic or therapeutic purposes.
Alpha-1-acid glycoprotein is an acute-phase plasma protein that seems primarily involved in modulating immune responses. It likely plays an important role in inflammatory processes and is known to bind various ligands, which may affect their distribution and availability. As a major serum protein, Orm1 is frequently studied for its contribution to immune modulation and its potential as a biomarker in research settings.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Rat Orm1 is a complex eukaryotic glycoprotein that requires precise folding, proper glycosylation, and specific post-translational modifications for its functional activity in the acute phase response. The E. coli expression system cannot provide the necessary eukaryotic glycosylation and complex folding environment required for this heavily glycosylated protein. While the protein may be soluble, it is highly unlikely to achieve the correct folding and glycosylation pattern needed for functional ligand-binding activity.
1. Antibody Development and Validation
Antibody development against linear epitopes is reliable, but glycosylation-dependent epitopes will not be represented. This recombinant Orm1 serves as an excellent immunogen for generating antibodies against linear epitopes of rat alpha-1-acid glycoprotein. However, antibodies may not efficiently recognize conformational epitopes or glycosylation-dependent epitopes on the native, properly glycosylated protein found in physiological conditions.
2. Biochemical Characterization
Basic biophysical characterization can be performed to assess the protein's physical properties. However, functional characterization studies will be limited due to the lack of glycosylation. Techniques like circular dichroism and size-exclusion chromatography can provide structural insights, but results will reflect a non-glycosylated form.
Final Recommendation & Action Plan
The E. coli expression system is fundamentally unsuitable for producing a functional version of this heavily glycosylated acute-phase protein. This non-glycosylated recombinant Orm1 is primarily suitable for developing linear epitope antibodies and basic biophysical characterization. Glycoprotein interactions require proper glycosylation and tertiary structure that E. coli cannot provide. Avoid interaction studies and functional studies entirely due to the critical importance of glycosylation for this protein's structure and function. For reliable Orm1 research, use mammalian expression systems that support proper glycosylation, or work with native protein purified from biological sources where correct glycosylation is preserved. Begin with basic biophysical characterization to understand the properties of this specific preparation, then proceed with linear epitope antibody development. For functional studies, alternative expression systems or native protein sources are essential.