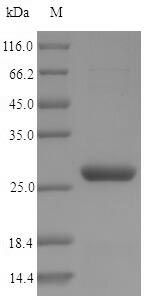
Recombinant Saccharomycerevisiae Nicotinamidase (PNC1) is expressed in E. coli and spans the complete protein sequence from amino acids 1 to 216. An N-terminal 6xHis-tag is attached to make purification and detection more straightforward. SDS-PAGE analysis shows the protein reaches greater than 90% purity, which appears to meet the standards needed for most research work.
Nicotinamidase—called PNC1 in Saccharomyces cerevisiae—seems to play an important role in the NAD+ salvage pathway. It converts nicotinamide to nicotinic acid. This conversion is likely essential for keeping cellular NAD+ levels stable, and these levels matter for numerous metabolic processes. Studies with nicotinamidase may offer insights into how cells handle metabolism and stress responses in yeast and possibly other organisms.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Based on the provided information, the recombinant Saccharomyces cerevisiae PNC1 is expressed in E. coli, a prokaryotic system that is generally suitable for producing soluble enzymatic proteins like nicotinamidase. PNC1 is a relatively small (216aa) metabolic enzyme that catalyzes the conversion of nicotinamide to nicotinic acid. While E. coli can often properly fold such enzymes, the eukaryotic origin of PNC1 and potential post-translational modifications may present folding challenges. The protein is full-length (1-216aa) with an N-terminal 6xHis tag and >90% purity. However, since activity is explicitly unverified, the protein cannot be assumed to be correctly folded or bioactive without experimental validation of its enzymatic activity using nicotinamide as a substrate.
1. Protein-Protein Interaction Studies Using His-Tag Affinity Purification
The N-terminal 6xHis tag enables technical feasibility for pull-down assays. However, if PNC1 is misfolded (a possibility in E. coli for a eukaryotic enzyme), it may not interact physiologically with true binding partners. Identified interactions could be non-physiological artifacts. This application should only be pursued after confirming proper folding and enzymatic activity.
2. Antibody Development and Validation
The recombinant PNC1 can serve as an effective immunogen for generating antibodies that recognize linear epitopes, even if the protein is misfolded. The high purity supports immunization protocols. However, antibodies may not recognize conformational epitopes of properly folded, native PNC1 in yeast cells.
3. Comparative Protein Structure and Stability Analysis
This application is well-suited for assessing the recombinant Saccharomyces cerevisiae PNC1 itself. Techniques like circular dichroism spectroscopy, thermal shift assays, and dynamic light scattering can evaluate the protein's folding state and stability. These studies are valuable even if the protein is inactive, as they characterize the recombinant Saccharomyces cerevisiae PNC1.
4. Substrate Specificity and Inhibitor Screening Assays
This application is highly dependent on correct folding and activity. If PNC1 is misfolded, substrate specificity studies and inhibitor screening will yield invalid results. These functional applications require prior validation of proper folding and demonstration of nicotinamidase activity.
Final Recommendation & Action Plan
Given the uncertainty in folding and bioactivity, recommend first validating the protein's enzymatic activity using a standard nicotinamidase assay with nicotinamide as substrate. Perform basic biophysical characterization (size-exclusion chromatography, circular dichroism) to assess folding quality. Antibody development can proceed as the lowest-risk application. Avoid all functional studies (interactions, kinetics, screening) until proper folding and activity are confirmed. For reliable PNC1 functional studies, consider including appropriate controls such as known substrates and validated inhibitors in experiments.