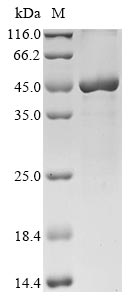
Recombinant Saccharomyces cerevisiae Sterol-4-alpha-carboxylate 3-dehydrogenase, decarboxylating (ERG26) is expressed in E. coli and contains the complete sequence spanning amino acids 1-349. The protein carries an N-terminal 10xHis-tag and a C-terminal Myc-tag, which streamline purification and detection processes. SDS-PAGE analysis indicates purity levels exceeding 90%, making it well-suited for rigorous research applications.
ERG26 appears to play a fundamental role in the ergosterol biosynthesis pathway—a process that seems critical for yeast and fungi survival. This enzyme catalyzes specific reactions in sterol intermediate conversion, ultimately contributing to ergosterol production. Since ergosterol is an essential membrane component in yeast cells, this protein may prove valuable for investigating sterol metabolism and could help researchers understand broader biological mechanisms in yeast systems.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Based on the provided information, the recombinant ERG26 protein is produced in E. coli with >90% purity by SDS-PAGE and contains the full-length sequence (1-349aa) with N-terminal 10×His and C-terminal Myc tags. However, no experimental data confirm proper folding or bioactivity. SDS-PAGE only assesses molecular weight and gross purity, not native conformation or enzymatic function. ERG26 is a eukaryotic enzyme requiring precise folding, potential cofactors (e.g., NAD⁺), and possibly interactions with other proteins for activity. The E. coli expression system lacks eukaryotic chaperones and post-translational modifications, increasing the risk of misfolding or inactivation. Without validation (e.g., circular dichroism for secondary structure, activity assays), the protein cannot be assumed to be correctly folded or bioactive.
1. Biochemical Characterization of Sterol Biosynthesis Pathway
This recombinant ERG26 protein could be used to study enzymatic kinetics and substrate preferences only if its folding and bioactivity are experimentally verified. The tags simplify purification, but misfolding would render kinetic data meaningless. Prior validation (e.g., activity assays with sterol substrates like 4β-methyl-4α-carboxy-cholesta-8,24-dien-3β-ol) is essential.
2. Protein-Protein Interaction Studies
The His/Myc tags enable technical feasibility for pull-down assays. However, misfolding could alter interaction interfaces, leading to false positives/negatives. Results must be cross-validated with native ERG26 from yeast or other systems.
3. Antibody Development and Validation
The full-length protein is suitable as an antigen for generating antibodies targeting linear epitopes, regardless of folding. However, antibodies may not recognize natively folded ERG26 if conformational epitopes are critical. Tags may also induce tag-specific antibodies.
4. Structural and Biophysical Analysis
The high purity makes the protein a candidate for structural studies (e.g., X-ray crystallography), but only if it is properly folded and monodisperse. Misfolded proteins are unsuitable, as they may form aggregates. Biophysical validation (e.g., size-exclusion chromatography, circular dichroism) is a prerequisite.
5. Enzyme Inhibitor Screening Assays
This application is entirely dependent on bioactivity confirmation. Without demonstrated enzymatic function, high-throughput screening for inhibitors (e.g., antifungal compounds like FR171456) would be ineffective. The tags facilitate immobilization, but functional integrity is paramount.
Final Recommendation & Action Plan
To ensure reliable outcomes, prioritize experimental validation of folding and bioactivity before any application. Recommended steps: (1) Perform enzyme activity assays using known substrates (e.g., 4α-carboxy sterols) and measure NAD⁺ dependence; (2) Use biophysical methods (e.g., circular dichroism, size-exclusion chromatography) to assess secondary structure and oligomeric state; (3) Include positive controls (e.g., native ERG26 from yeast). If validation fails, repurpose the protein for non-functional uses (e.g., antibody generation). Always disclose the lack of functional data in publications to avoid misinterpretation.