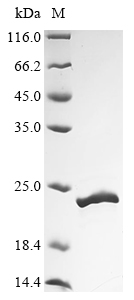
Recombinant Staphylococcus aureus Chemotaxis inhibitory protein (chp) is expressed in E. coli and consists of the full-length mature protein, covering amino acids 29 to 149. The protein comes with an N-terminal 10xHis-tag and a C-terminal Myc-tag to make purification and detection more straightforward. SDS-PAGE analysis shows the product has a purity greater than 90%, which appears suitable for detailed research studies.
The chemotaxis inhibitory protein from S. aureus seems to play a role in modulating host immune responses. It likely works by interfering with chemotactic signaling pathways. This protein has become important for understanding how bacteria evade immune responses and manipulate host immunity, which is why it's drawing attention in microbial pathogenesis and immunology research.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Based on the provided information, the folding state and bioactivity of this recombinant CHP protein are unverified and cannot be assumed. The protein is expressed in E. coli as the mature protein fragment (29-149aa) with both N- and C-terminal tags, which could potentially interfere with the correct folding required for its biological activity (inhibiting chemotaxis). The high purity (>90%) confirms a lack of significant contaminants but does not provide evidence of a proper three-dimensional structure. Therefore, any application that depends on the protein's specific biological function (e.g., binding to its natural targets) is speculative without experimental validation.
1. Antibody Development and Immunoassay Studies
This recombinant CHP protein is suitable for use as an immunogen to generate specific antibodies. The dual-tag system provides flexible options for detection and purification during antibody screening. The >90% purity is adequate for immunization protocols and subsequent characterization (e.g., by ELISA). However, antibodies generated will primarily recognize linear epitopes or the tags themselves. Their ability to bind the natively folded, functional CHP protein in a biological context is not guaranteed and would need to be confirmed.
2. Protein-Protein Interaction Studies
The dual tags make this protein practical for pull-down or co-immunoprecipitation assays to identify potential binding partners. However, a critical caveat is that any interaction detected is entirely contingent on the recombinant CHP protein being correctly folded. A negative result in such an assay could be due to a lack of genuine binding partners or, equally, due to the bait protein being misfolded and inactive.
3. Biochemical Characterization and Stability Studies
This recombinant CHP protein is well-suited for biochemical and biophysical characterization, including studies of thermal stability, pH tolerance, and protease resistance. The high purity is beneficial for techniques like circular dichroism spectroscopy to analyze secondary structure. The tags offer detection methods. This application is largely independent of the protein's native bioactivity, as it focuses on intrinsic physicochemical properties.
4. Competitive Binding Assays
The recombinant CHP protein could be used in competitive binding assays only if its bioactivity is first confirmed. The tags facilitate immobilization or detection. Using this unvalidated protein in such assays risks generating misleading data, as an inability to compete could be misinterpreted as a lack of binding affinity rather than a lack of activity in the recombinant protein itself.
Final Recommendation & Action Plan
The immediate and necessary priority is to establish a functional assay to confirm the protein's bioactivity, such as its ability to inhibit neutrophil or leukocyte chemotaxis in a cell-based assay, before proceeding with applications that rely on specific biological interactions (Applications 2 and 4). A positive result would validate its use in functional studies, while a negative result would limit its utility to structural/biophysical characterization (Application 3) and as an immunogen for generating linear-epitope antibodies (Application 1). Until functional activity is demonstrated, data from binding or interaction studies should be interpreted with extreme caution.