[1] Lee, Anna K., and Patrick Ryan Potts. "A comprehensive guide to the MAGE family of ubiquitin ligases." Journal of molecular biology 429.8 (2017): 1114-1142.
[2] Chomez, Patrick, et al. "An overview of the MAGE gene family with the identification of all human members of the family." Cancer research 61.14 (2001): 5544-5551.
[3] Weon, Jenny L., and Patrick Ryan Potts. "The MAGE protein family and cancer." Current opinion in cell biology 37 (2015): 1-8.
[4] Meek, David W., and Lynnette Marcar. "MAGE-A antigens as targets in tumour therapy." Cancer letters 324.2 (2012): 126-132.
[5] Sang, Meixiang, et al. "MAGE-A family: attractive targets for cancer immunotherapy." Vaccine 29.47 (2011): 8496-8500.
[6] Aubry, Florence, et al. "MAGE‐A4, a germ cell specific marker, is expressed differentially in testicular tumors." Cancer: Interdisciplinary International Journal of the American Cancer Society 92.11 (2001): 2778-2785.
[7] Hagiwara, Yoshio, et al. "Consequences of point mutations in melanoma-associated antigen 4 (MAGE-A4) protein: Insights from structural and biophysical studies." Scientific reports 6.1 (2016): 25182.
[8] Sun, Qian, et al. "T-cell receptor gene therapy targeting melanoma-associated antigen-A4 by silencing of endogenous TCR inhibits tumor growth in mice and human." Cell death & disease 10.7 (2019): 475.
[9] Chambost, Hervé, et al. "Expression of gene MAGE-A4 in Reed-Sternberg cells." Blood, The Journal of the American Society of Hematology 95.11 (2000): 3530-3533.
[10] Kocher, Thomas, et al. "Prognostic relevance of MAGE‐A4 tumor antigen expression in transitional cell carcinoma of the urinary bladder: a tissue microarray study." International journal of cancer 100.6 (2002): 702-705.
[11] Gao, Yanzhe, et al. "A neomorphic cancer cell-specific role of MAGE-A4 in trans-lesion synthesis." Nature communications 7.1 (2016): 12105.
[12] Saito, Takuro, et al. "High expression of MAGE-A4 and MHC class I antigens in tumor cells and induction of MAGE-A4 immune responses are prognostic markers of CHP-MAGE-A4 cancer vaccine." Vaccine 32.45 (2014): 5901-5907.
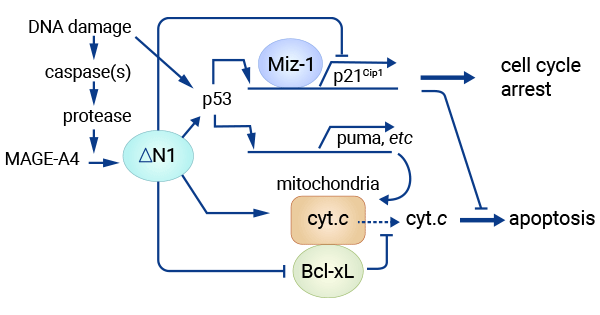
[13] Sakurai, Toshiharu, et al. "A cleaved form of MAGE-A4 binds to Miz-1 and induces apoptosis in human cells." Journal of Biological Chemistry 279.15 (2004): 15505-15514.
[14] Cruz, Conrad R., et al. "Improving T-cell therapy for relapsed EBV-negative Hodgkin lymphoma by targeting upregulated MAGE-A4." Clinical Cancer Research 17.22 (2011): 7058-7066.
[15] Sanderson, Joseph P., et al. "Preclinical evaluation of an affinity-enhanced MAGE-A4-specific T-cell receptor for adoptive T-cell therapy." Oncoimmunology 9.1 (2020): 1682381.
[16] Takahashi, Norihiko, et al. "First clinical trial of cancer vaccine therapy with artificially synthesized helper/killer‐hybrid epitope long peptide of MAGE‐A4 cancer antigen." Cancer science 103.1 (2012): 150-153.
[17] Kageyama, Shinichi, et al. "Adoptive transfer of MAGE-A4 T-cell receptor gene-transduced lymphocytes in patients with recurrent esophageal cancer." Clinical Cancer Research 21.10 (2015): 2268-2277.
[18] Okumura, Satoshi, et al. "Chimeric antigen receptor T-cell therapy targeting a MAGE A4 peptide and HLA-A* 02: 01 complex for unresectable advanced or recurrent solid cancer: protocol for a multi-institutional phase 1 clinical trial." BMJ open 12.11 (2022): e065109.
[19] Kobayashi, Hiroshi, et al. "The biology of uterine sarcomas: a review and update." Molecular and clinical oncology 1.4 (2013): 599-609.
[20] Tsai, Jong-Rung, et al. "Differential expression profile of MAGE family in non-small-cell lung cancer." Lung cancer 56.2 (2007): 185-192.
[21] Dhodapkar, Madhav V., et al. "Expression of cancer/testis (CT) antigens MAGE-A1, MAGE-A3, MAGE-A4, CT-7, and NY-ESO-1 in malignant gammopathies is heterogeneous and correlates with site, stage and risk status of disease." Cancer Immunity 3.1 (2003).
[22] Cabezon, Teresa, et al. "Proteomic profiling of triple-negative breast carcinomas in combination with a three-tier orthogonal technology approach identifies Mage-A4 as potential therapeutic target in estrogen receptor negative breast cancer." Molecular & Cellular Proteomics 12.2 (2013): 381-394.
[23] Two-Pronged, A. "471. CMV-Specific HER2-Redirected T Cells for the Adoptive Immunotherapy of Glioblastoma." Molecular Therapy 18 (2010): 1.
[24] Guo, Liru, et al. "The expression and clinical significance of melanoma‑associated antigen‑A1,‑A3 and‑A11 in glioma." Oncology letters 6.1 (2013): 55-62.
[25] Ebrahimabadi, S., et al. "MAGE-A4-CAR-NK CELL THERAPY FOR THE TREATMENT OF SOLID TUMORS." Hematology, Transfusion and Cell Therapy 45 (2023): S534-S535.
[26] Chen, Xiaohua, et al. "Analysis of the function of MAGE-A in esophageal carcinoma by bioinformatics." Medicine 98.21 (2019).
[27] Ueda, Shugo, et al. "NY-ESO-1 antigen expression and immune response are associated with poor prognosis in MAGE-A4-vaccinated patients with esophageal or head/neck squamous cell carcinoma." Oncotarget 9.89 (2018): 35997.
[28] Peikert, Tobias, et al. "Melanoma antigen A4 is expressed in non–small cell lung cancers and promotes apoptosis." Cancer research 66.9 (2006): 4693-4700.
[29] Liu, Fangfang, et al. "A study of protein expression of MAGE-A3, MAGE-A4 and MAGE-A10 genes in colorectal carcinoma and its clinical significance." Chinese Journal of General Surgery (2012): 37-39.
[30] Baba, Tetsuro, et al. "Clinical significance of human leukocyte antigen loss and melanoma-associated antigen 4 expression in smokers of non-small cell lung cancer patients." International journal of clinical oncology 18 (2013): 997-1004.
[31] Zhang, Yujie, Yuxin Zhang, and Li Zhang. "Expression of cancer–testis antigens in esophageal cancer and their progress in immunotherapy." Journal of cancer research and clinical oncology 145 (2019): 281-291.
[32] Bergeron, Alain, et al. "High frequency of MAGE‐A4 and MAGE‐A9 expression in high‐risk bladder cancer." International Journal of Cancer 125.6 (2009): 1365-1371.

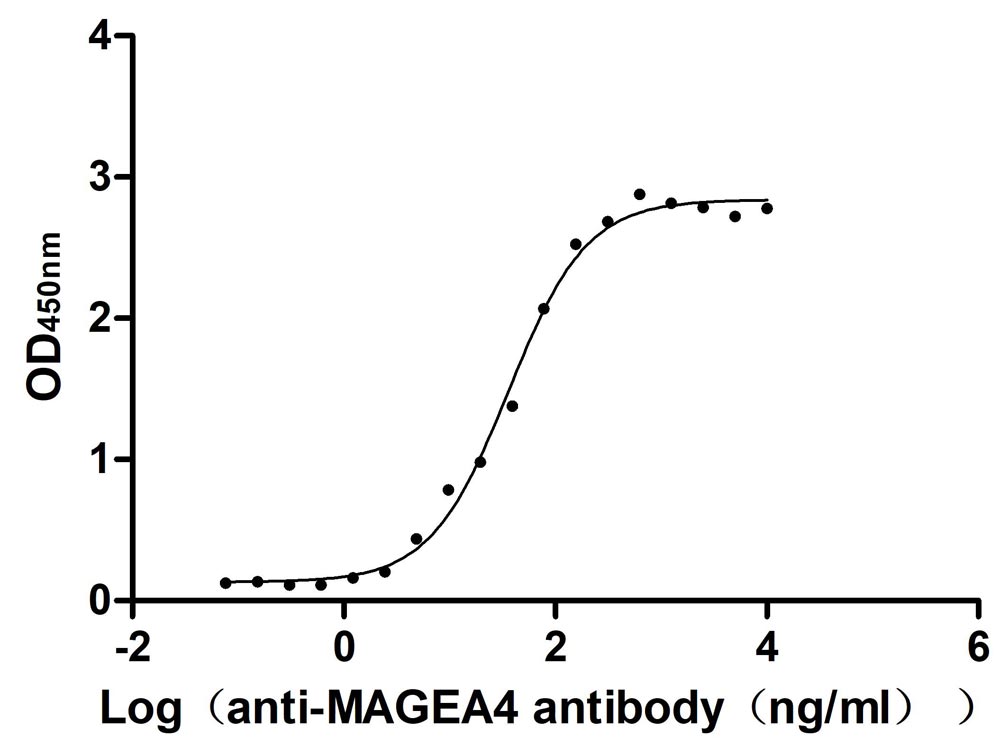
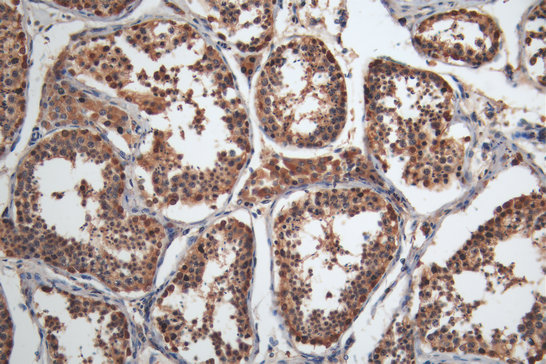
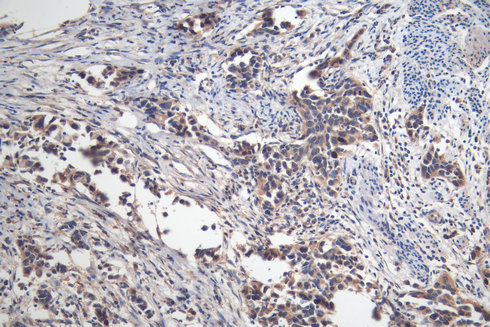
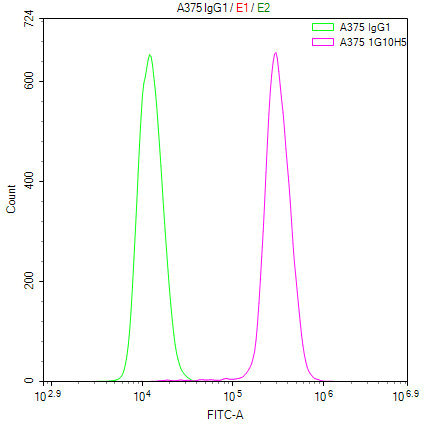


Comments
Leave a Comment