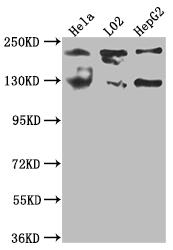
The MGEA5 gene encodes protein O-GlcNAcase (OGA), which is responsible for the hydrolysis of the O-linked β-N-acetyl glucosamine (O-GlcNAc) modification, an essential protein glycosylation event that modulates the function of numerous cellular proteins in response to nutrients and stress. OGA is a member of the family of hexosaminidases and is mainly localized in the cytosol. However, unlike lysosomal hexosaminidases, OGA activity is the highest at neutral pH (approximately 7). Nicholas B. Hastings et al. has demonstrated that inhibition of O-GlcNAcase leads to elevation of O-GlcNAc tau and reduction of tauopathy and cerebrospinal fluid tau in rTg4510 mice.
Compared with the polyclonal and monoclonal antibodies of MGEA5, this MGEA5 recombinant antibody has the features of increased reproducibility and control, animal-free technology, high degree of monovalency, high batch-to-batch consistency, easier isotype conversion, etc. And it has been validated in ELISA, WB.