PABPN1 is a multifactorial mRNA processing regulator that controls muscle wasting and atrophy. PABPN1 activates polydenylate polymerase and regulates the length of the poly(A) tail on RNA transcripts. PABPN1 influences mRNA levels and stability by regulating the usage of alternative polyadenylation sites. PABPN1 is also involved in the processing of long non-coding RNA and short nucleolar RNA, as well as nuclear surveillance, which results in RNA hyperadenylation and degradation. PABPN1 levels in humans are lowered from midlife onwards, particularly in skeletal muscles. Oculopharyngeal muscular dystrophy (OPMD), a late-onset myopathy, is caused by an expansion mutation in PABPN1. Reduced PABPN1 levels cause muscle wasting and atrophy.
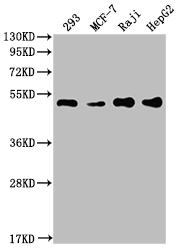
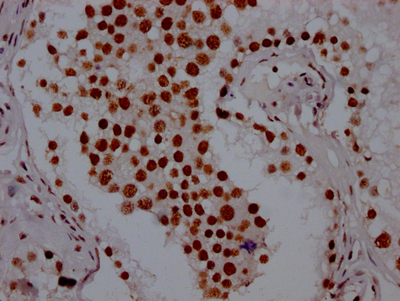
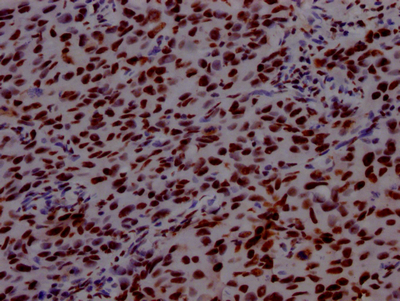
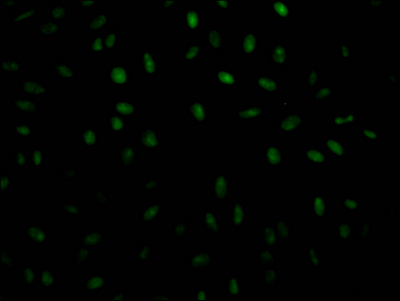
The generation of the recombinant PABPN1 antibody includes obtaining the PABPN1 antibody gene, cloning the gene into a plasma vector, introducing the recombinant vector into mammalian cell lines, and achieving expression of adequate amounts of functional antibody. The recombinant PABPN1 antibody was purified using A synthesized peptide derived from human PABPN1. It is reactive with the PABPN1 protein from Human and is suitable for the use in the ELISA, WB, IHC, IF, FC.