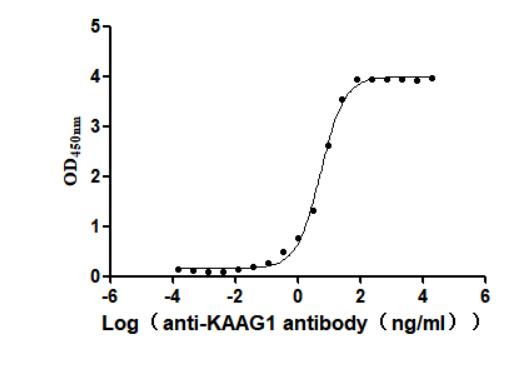
This active recombinant human KAAG1 protein is produced using a baculovirus expression system and includes amino acids 1 to 84 of the full-length KAAG1 sequence. It features a C-terminal 10xHis tag, allowing for efficient purification and detection. Supplied as a lyophilized powder, the recombinant KAAG1 protein has a purity level exceeding 90%, as verified by SDS-PAGE. Endotoxin content is strictly maintained below 1.0 EU/μg based on LAL testing, ensuring suitability for sensitive biological applications. Functional validation was performed using a binding ELISA: when coated at 2 μg/mL, the KAAG1 protein specifically interacts with the anti-KAAG1 recombinant antibody (CSB-RA871385MA1HU), with an EC50 measured between 4.924 and 6.034 ng/mL. This confirms its biological activity and utility in antibody-binding assays.
Human HKAA1 is a distinct protein associated with kidney function and pathology. This antigen has garnered attention primarily with autoimmune diseases and renal transplant immunology. It signifies an important component in the immune response, particularly in conditions like systemic lupus erythematosus (SLE), where kidney involvement is prevalent.
The immunological studies indicate a role for renal-associated autoantigens, such as HKAA1, in the pathogenesis of nephritis contributing to conditions like glomerulonephritis, seen with systemic autoimmune diseases [1]. This antigen can serve as a target for autoantibodies, which facilitate kidney damage through various mechanisms, likely involving immune complex deposition in renal tissues and subsequent inflammatory responses. For instance, research has shown that certain autoantibodies can become activated upon interaction with kidney antigens, leading to complement activation and injury to the kidney's structural components [2].
Moreover, studies suggest that antigen presentation plays an essential role in kidney injury, emphasizing the importance of identifying specific kidney antigens such as HKAA1. This could provide insights into immunological responses contributing to conditions like transplant rejection and chronic graft dysfunction where antigen-antibody interactions significantly contribute to allograft outcomes [3][4]. The presence of donor-specific antibodies, which can target such antigens, corresponds with adverse transplant outcomes, reinforcing the role of kidney-associated antigens in post-transplant complications [5].
Furthermore, HKAA1 is relevant in the context of pediatric populations experiencing proteinuria and renal insufficiency post-transplant, indicating its potential as a biomarker for monitoring transplant outcomes [6]. The role of HKAA1 in inducing specific immune responses highlights the antigen's potential utility in diagnostic practices aimed at identifying individuals who may be at risk for renal diseases or transplant complications.
References:
[1] D. Koffler, P. Schur, & H. Kunkel. Immunological studies concerning the nephritis of systemic lupus erythematosus. The Journal of Experimental Medicine, vol. 126, no. 4, p. 607-624, 1967. https://doi.org/10.1084/jem.126.4.607
[2] A. Cybulsky, R. Quigg, & D. Salant. Experimental membranous nephropathy redux. Ajp Renal Physiology, vol. 289, no. 4, p. F660-F671, 2005. https://doi.org/10.1152/ajprenal.00437.2004
[3] S. Yu, H. Huh, et al. Pre-transplant angiotensin ii type 1 receptor antibodies and anti-endothelial cell antibodies predict graft function and allograft rejection in a low-risk kidney transplantation setting. Annals of Laboratory Medicine, vol. 40, no. 5, p. 398-408, 2020. https://doi.org/10.3343/alm.2020.40.5.398
[4] M. Gniewkiewicz, K. Czerwińska, K. Zielniok, & M. Durlik. Impact of resolved preformed, persistent preformed, and de novo anti-hla donor-specific antibodies in kidney transplant recipients on long-term renal graft outcomes. Journal of Clinical Medicine, vol. 12, no. 10, p. 3361, 2023. https://doi.org/10.3390/jcm12103361
[5] S. Sethi, J. Choi, M. Toyoda, A. Vo, A. Peng, & S. Jordan. Desensitization: overcoming the immunologic barriers to transplantation. Journal of Immunology Research, vol. 2017, p. 1-11, 2017. https://doi.org/10.1155/2017/6804678
[6] J. Goodwin, M. Palmer, F. Pashankar, A. Tufró, & G. Moeckel. A 7-year-old boy with renal insufficiency and proteinuria after stem cell transplant for t-cell acute lymphoblastic leukemia. Clinical Nephrology, 2013. https://doi.org/10.5414/cn107767