Recombinant Human Tetraspanin-8 (TSPAN8)-VLPs are produced using a mammalian cell expression system, which appears to preserve the protein's native conformation and post-translational modifications. The protein spans the full length from amino acids 1 to 237. A C-terminal 10xHis-tag is included, though it can only be assessed under denaturing conditions. These virus-like particles show biological activity - their binding ability has been demonstrated in functional ELISA experiments. Endotoxin levels stay below 1.0 EU/µg, as confirmed by the LAL method.
Tetraspanin-8 belongs to the tetraspanin family, which is characterized by four transmembrane domains. This protein likely plays an important role in cellular processes like signal transduction, cell adhesion, and migration. Tetraspanins seem to be involved in organizing multimolecular membrane complexes and may influence various signaling pathways. This makes TSPAN8 potentially valuable for research into cellular communication and membrane dynamics.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
1. Membrane Protein-Antibody Interaction Studies
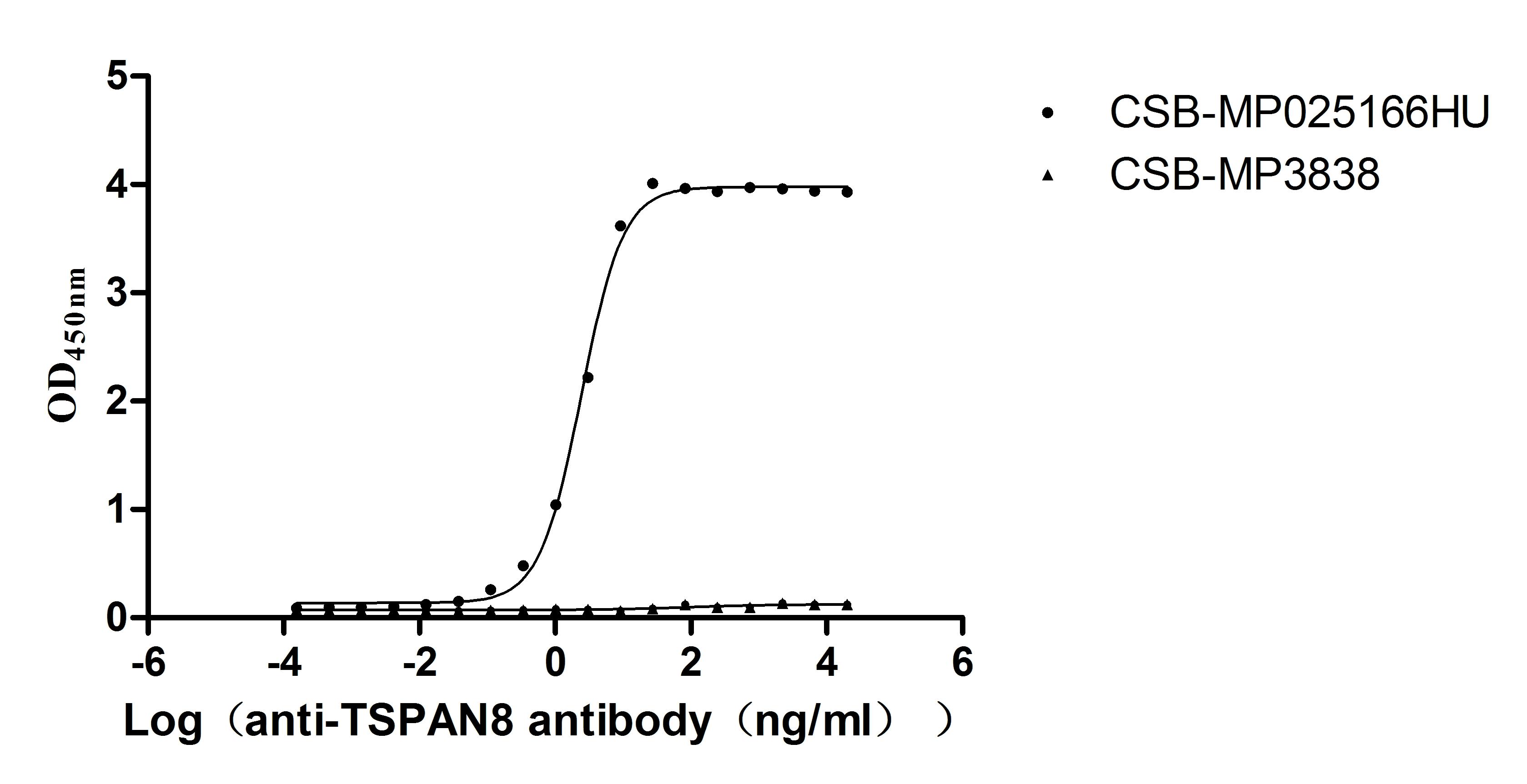
The confirmed binding activity of TSPAN8-VLPs with anti-TSPAN8 antibody (EC50: 2.261-2.623 ng/mL) in a functional ELISA directly supports its use for antibody interaction studies. The VLP presentation maintains a native-like membrane environment, making it suitable for evaluating antibody kinetics, specificity, and epitope accessibility in a physiological context.
2. Membrane Protein Complex Formation Assays
The VLP format provides a lipid bilayer that may support native protein-protein interactions for TSPAN8. However, the provided activity data only confirm antibody binding, not interactions with other membrane proteins. This application could be explored, but results should be validated with additional assays to ensure physiological relevance.
3. Vaccine Antigen Development Research
This application is theoretically possible but may not be optimal. While the VLP platform mimics viral structures and TSPAN8 is correctly folded, TSPAN8 is not a typical vaccine target (it is a self-protein involved in cell adhesion). Its use in vaccine research could risk autoimmune responses. It should be refined to focus on basic immunogenicity studies rather than vaccine development, emphasizing caution for in vivo applications.
4. Membrane Protein Purification Method Development
The C-terminal 10xHis tag is noted to be "only functional under denaturing conditions," but VLPs require non-denaturing conditions to maintain integrity. Therefore, the His tag may not be accessible for standard purification methods without disrupting the VLP structure. This protein is not suitable for purification method development; instead, it should be used as a model for studying VLP stability or binding assays under native conditions.
Final Recommendation & Action Plan
Given the confirmed bioactivity and mammalian cell expression, this TSPAN8-VLP product is well-suited for antibody interaction studies and membrane protein research in a native-like environment. Prioritize applications 1 and 2, but for protein complex assays, validate interactions with complementary methods. Avoid vaccine development due to potential safety concerns, and do not use for purification optimization given the tag limitations. Always include the negative control VLPs in experiments to ensure specificity. For future work, consider functional assays to expand beyond antibody binding, such as probing interactions with natural ligands or other tetraspanins.