Introducing Recombinant Human VAPB, a high-quality protein engineered for signal transduction research. This partial protein spans the 2-132aa expression region of Vesicle-associated membrane protein-associated protein B/C (VAMP-B/VAMP-C), a critical component in cellular communication and regulation. Produced in E. coli and featuring a C-terminal 6xHis-tag for convenient purification, Recombinant Human VAPB offers a reliable and effective solution for researchers seeking to study VAMP-B/VAMP-C protein.
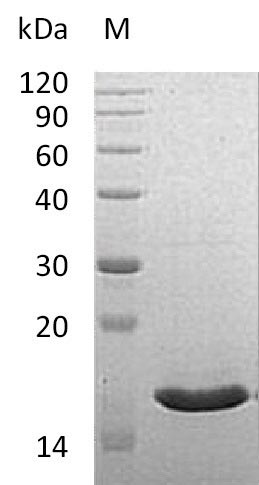
Our Recombinant Human VAPB maintains a purity of greater than 90%, as determined by SDS-PAGE, ensuring that it will deliver consistent performance in your experiments. Moreover, the endotoxin levels are less than 1.0 EU/µg, as measured by the LAL method, ensuring minimal interference with your research. The biological activity of this protein is confirmed by its ability to bind Human EphB2 in functional ELISA, with an ED50 of less than 20 µg/ml, demonstrating a high level of activity.
Available as a lyophilized powder, Recombinant Human VAPB provides convenient storage and use for various experimental designs. Choose this protein for a dependable and effective resource for your signal transduction research needs.