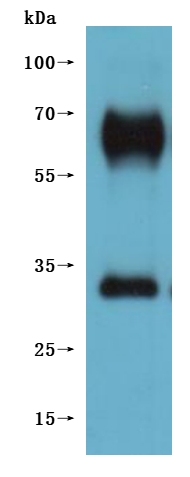
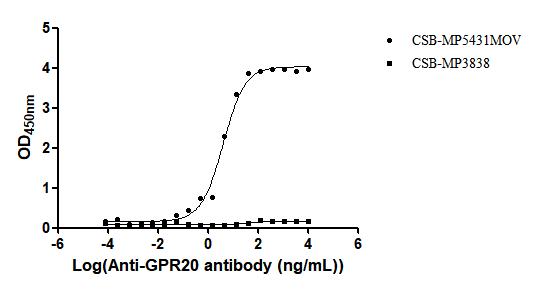
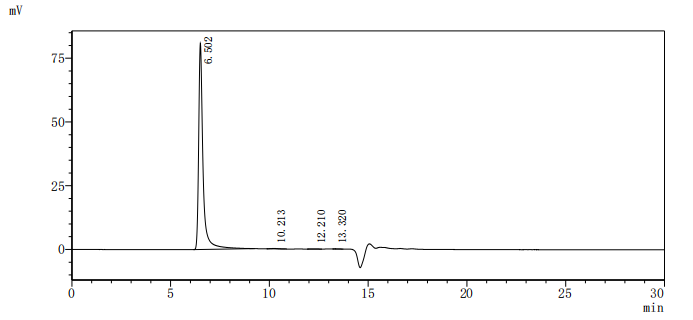
The recombinant Macaca fascicularis GPR20 protein is produced as a virus-like particle (VLP), enabling it to maintain a membrane-associated conformation that closely mimics its native state. Expressed in mammalian cells, this construct includes the full-length GPR20 sequence (amino acids 1–359) and features a C-terminal 10xHis tag for purification and analytical use. The recombinant GPR20 protein is supplied in lyophilized form and demonstrates high purity, exceeding 95% as determined by SEC-HPLC. Functional validation in an ELISA assay confirms its ability to bind the anti-GPR20 recombinant antibody (CSB-RA860774MA1HU) when immobilized at 10 μg/mL, with an EC50 ranging from 3.549 to 6.542 ng/mL. The specificity of the assay is supported by a negative VLP control (CSB-MP3838). With its preserved structural integrity and confirmed activity, this GPR20 VLP is a reliable reagent for studies involving G protein-coupled receptor function, antibody screening, or therapeutic development.
The GPR20 protein in Macaca fascicularis, commonly known as the cynomolgus macaque, is part of a broader category of GPCRs. These receptors play essential roles in various physiological processes by mediating signal transduction, particularly in response to extracellular stimuli. While specific studies directly focusing on GPR20 in Macaca fascicularis are limited, we can draw from the general characteristics of GPCRs and their relevance in the context of primate biology and health, particularly concerning metabolic processes and disease models.
GPCRs typically operate through G proteins to facilitate intracellular signaling pathways pivotal for regulating multiple physiological functions such as hormone responses, sensory perception, and immune responses. In primates, including Macaca fascicularis, the variety of GPCRs, including orphan receptors such as GPR20, raises significant interest in their potential roles in disease mechanisms and drug responses, especially as cynomolgus macaques serve as vital models for biomedical research [1][2].
Recent research indicates that GPCRs, and potentially GPR20, are associated with metabolic alterations as observed in studies involving macaque models for diabetes and metabolic syndrome. These conditions are critical areas of study given their parallels to human physiology and disease [3]. Moreover, the variations in GPCR signaling pathways among different primate species can provide insights into evolutionary adaptations and functional discrepancies that influence drug metabolism and therapeutic responses in humans [4].
Alongside these metabolic implications, various studies emphasize the importance of the cynomolgus macaque as a representative model for assessing neurological disorders. Research surrounding GPCR interactions—including that possibly involving GPR20—may elucidate new pathways for understanding complex interactions in neurodegenerative diseases, which affect both human and primate health. For instance, the involvement of GPCRs in dopamine signaling pathways has been studied extensively within MPTP-induced parkinsonian macaque models, demonstrating the intricate relationships between receptor signaling, brain function, and behavioral outcomes [5].
References:
[1] J. Estes, S. Wong, & J. Brenchley. Nonhuman primate models of human viral infections. Nature Reviews Immunology, vol. 18, no. 6, p. 390-404, 2018. https://doi.org/10.1038/s41577-018-0005-7
[2] S. Kanthaswamy, J. Ng, et al. The genetic composition of populations of cynomolgus macaques (macaca fascicularis) used in biomedical research. Journal of Medical Primatology, vol. 42, no. 3, p. 120-131, 2013. https://doi.org/10.1111/jmp.12043
[3] S. Laila, D. Astuti, I. Suparto, E. Handharyani, T. Register, & D. Sajuthi. Atherosclerotic lesion of the carotid artery in indonesian cynomolgus monkeys receiving a locally sourced atherogenic diet. Veterinary Sciences, vol. 9, no. 3, p. 105, 2022. https://doi.org/10.3390/vetsci9030105
[4] S. Pérez, M. Vernica, & O. Rascol. Ayurveda medicine for the treatment of parkinson’s disease. International Journal of Integrative Medicine, p. 1, 2013. https://doi.org/10.5772/56251
[5] L. Tian, Y. Xia, H. Flores, M. Campbell, S. Moerlein, & J. Perlmutter. Neuroimaging analysis of the dopamine basis for apathetic behaviors in an mptp-lesioned primate model. Plos One, vol. 10, no. 7, p. e0132064, 2015. https://doi.org/10.1371/journal.pone.0132064