In the field of cancer therapy, overcoming drug resistance remains a significant challenge for researchers. GPR20, a long-silent "orphan" GPCR, is emerging into the spotlight due to its unique profile in drug-resistant gastrointestinal stromal tumors (GIST). This article will delve into how GPR20 has transitioned from its mysterious "orphan" status to becoming a promising new target against drug-resistant GIST, revealing the underlying signaling mechanisms and the latest breakthroughs in targeted drug development, offering new hope for future cancer treatment.
1. GPR20 – An Emerging G Protein-Coupled Receptor Target
G protein-coupled receptors (GPCRs) are the largest family of cell membrane receptors, involved in numerous physiological processes and representing important drug targets. Among these receptors, approximately 140 members are known as orphan GPCRs, whose endogenous ligands remain unknown, holding immense potential for drug development. GPR20, as an emerging class A orphan GPCR, has gradually attracted widespread attention due to its unique constitutive activity and potential roles in various physiological and pathological processes [1].
Studies show that GPR20 constitutively activates Gi/o proteins, leading to reduced intracellular cyclic adenosine monophosphate (cAMP) levels and potentially inhibiting cell proliferation by regulating cell cycle signaling. The molecular mechanism behind its high basal activity has been elucidated using cryo-electron microscopy (cryo-EM), revealing that its unique N-terminal helical cap structure plays a key role in activation, and unassigned electron density in the orthosteric pocket suggests the presence of an endogenous ligand [1].
In the disease realm, GPR20 has been identified as a novel non-kinase therapeutic target in gastrointestinal stromal tumors (GIST), particularly showing differentially high expression in GIST resistant to tyrosine kinase inhibitors (TKIs) [2]. Based on this, the GPR20-targeting antibody-drug conjugate (ADC) DS-6157a was developed and demonstrated GPR20 expression-dependent anti-tumor activity in preclinical models, showing efficacy against TKI-resistant GIST [2].
2. Background and Initial Identification of GPR20
As an orphan GPCR, GPR20 was initially discovered via polymerase chain reaction (PCR) on genomic DNA. Its sequence analysis showed high homology with nucleotide or lipid receptors, but early studies had not identified its endogenous ligand or physiological function [1]. The constitutive activity of GPR20 makes it an intriguing subject of study; even in the absence of a known ligand, GPR20 can persistently activate Gi/o proteins, thereby influencing cAMP levels [2]. Further research indicates that GPR20, through Gi/o protein activation, inhibits adenylate cyclase (AC) activity, thus reducing cAMP levels, which may subsequently affect cell proliferation and other physiological functions [2].
3. Research Mechanisms and Signaling Pathways of GPR20
GPR20 exhibits significant constitutive activity, meaning it can activate its coupled G proteins even in the absence of a known ligand. This characteristic endows it with a unique role in cell physiology.This section will explore in depth how GPR20 regulates downstream signaling via the Gi/o protein pathway, particularly its inhibitory effect on cAMP levels and its impact on cell proliferation [2].
3.1 Constitutive Activity of GPR20 and Gi/o Protein Coupling
The constitutive activity of GPR20 has been validated in multiple cell lines. For instance, in HEK293 cells, GPR20 overexpression significantly reduces cAMP levels while increasing GTPγS binding, indicating that GPR20 can spontaneously activate Gi/o proteins [2]. This mechanism can be inhibited by pertussis toxin (PTX), further confirming the crucial role of Gi/o proteins. Experiments also found that the DRY motif in GPR20 is essential for G protein activation [2].
3.2 Structural Biology Insights into GPR20
Recently, using cryo-electron microscopy (cryo-EM), researchers successfully resolved the structure of the GPR20-Gi protein complex, revealing the molecular mechanism underlying its constitutive activity [1]. Among the findings, the unique N-terminal helical cap structure of GPR20 stabilizes its active conformation, allowing GPR20 to persistently activate Gi/o proteins without a ligand [1]. Furthermore, structural analysis revealed unassigned electron density in the orthosteric pocket of GPR20, suggesting the potential presence of an endogenous ligand or important regulatory molecule [1].
3.3 Cellular Functions and Ligand Exploration of GPR20
Constitutive activation of GPR20 affects intracellular cAMP levels and mitogenic signaling, inhibiting cell proliferation. Studies also suggest that the high basal activity of GPR20 might be key to its role in cell physiology. Further exploration to identify its endogenous ligand will provide important clues for understanding the biological functions of GPR20 [1].
4. GPR20 and Associated Diseases: The Case of Gastrointestinal Stromal Tumor (GIST)
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor of the gastrointestinal tract, and its treatment primarily relies on tyrosine kinase inhibitors (TKIs). However, TKI resistance is a major cause of GIST treatment failure. Therefore, finding new targets is of great significance for overcoming TKI resistance [3].
4.1 The Role of GPR20 in GIST
The high expression of GPR20 in GIST makes it a potential therapeutic target. Research indicates that GPR20 expression is significantly increased in GIST cell lines and patient-derived xenograft (PDX) models [3]. Based on this discovery, researchers developed the GPR20-targeting antibody-drug conjugate (ADC) DS-6157a, which demonstrated GPR20 expression-dependent anti-tumor activity in preclinical models and was effective against TKI-resistant GIST [3].
4.2 Preclinical Studies of GPR20 in GIST
Preclinical studies of DS-6157a showed significant anti-tumor activity in various GIST models, particularly exhibiting excellent efficacy against GIST models resistant to imatinib, sunitinib, and regorafenib [3]. Additionally, the pharmacokinetic properties of DS-6157a have been preliminarily evaluated in early clinical studies [4].
5. Latest Research Progress on GPR20-Targeting Drugs
DS-6157a represents the first antibody-drug conjugate (ADC) targeting GPR20, designed to overcome GIST resistance to TKI therapy. This drug consists of an anti-GPR20 antibody, a cleavable linker, and the topoisomerase I inhibitor payload DXd. It exerts anti-tumor effects in a GPR20 expression-dependent manner in GIST xenograft models [3].
Preclinical evaluation of DS-6157a demonstrated significant anti-tumor activity against TKI-resistant GIST models. Furthermore, early clinical trials indicated a clear relationship between the pharmacokinetic properties and the safety profile of DS-6157a, with drug exposure correlated with hematological adverse events such as neutropenia and thrombocytopenia [4].
6. GPR20 Research Tools
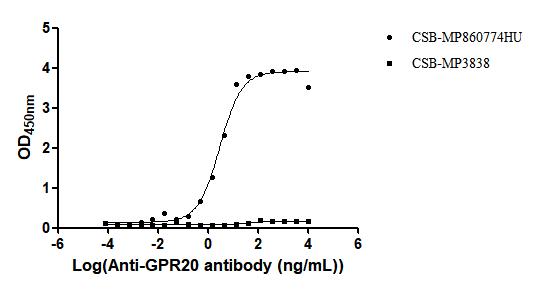
As an orphan GPCR, the constitutive activity of GPR20 and its potential in diseases like GIST have driven research into targeted therapies for this receptor. Although current research is still in its early stages, novel drugs like DS-6157a have demonstrated the great potential of GPR20 as a therapeutic target. Cusabio provides GPR20 recombinant proteins and antibody products to assist you in further exploring the endogenous ligand of GPR20 and its role in more diseases, promoting the clinical application of GPR20-targeted therapies.
References
[1] Xi Lin, Shan Jiang, Yiran Wu, Xiaohu Wei, G. Han, Lijie Wu, Junlin Liu, Bo Chen, Zhibin Zhang, Suwen Zhao, V. Cherezov, Fei Xu.(2023). The activation mechanism and antibody binding mode for orphan GPR20.
[2] Momoko Hase, T. Yokomizo, Takao Shimizu, Motonao Nakamura.(2008). Characterization of an Orphan G Protein-coupled Receptor, GPR20, That Constitutively Activates Gi Proteins*.
[3] K. Iida, A. A. Abdelhamid Ahmed, A. Nagatsuma, T. Shibutani, Satoru Yasuda, Michiko Kitamura, C. Hattori, Manabu Abe, J. Hasegawa, Takuma Iguchi, Tsuyoshi Karibe, T. Nakada, K. Inaki, Reiko Kamei, Yuki Abe, T. Nomura, J. Andersen, S. Santagata, Matthew L. Hemming, S. George, T. Doi, A. Ochiai, G. Demetri, T. Agatsuma.(2021). Identification and Therapeutic Targeting of GPR20, Selectively Expressed in Gastrointestinal Stromal Tumors, with DS-6157a, a First-In-Class Antibody-Drug Conjugate.
[4] Brittany P Tran, Maura Fallon, Kristin Follman, Sergio Iadevaia, Madan Kundu, A. Laadem, Yvonne Lau, Satoshi Nishioka, Julia Shi, Xinyuan Zhang. Population pharmacokinetics and exposure-safety of DS-6157a in patients with advanced gastrointestinal stromal tumor (GIST).
CUSABIO team. GPR20: From "Orphan" to a Promising Novel Target for Overcoming Drug-Resistant GIST. https://www.cusabio.com/c-21274.html

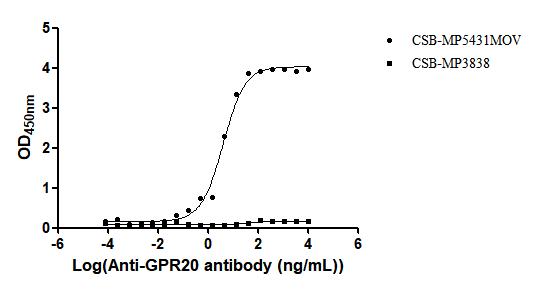
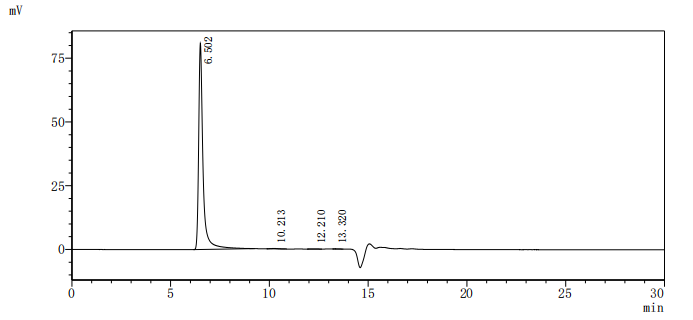
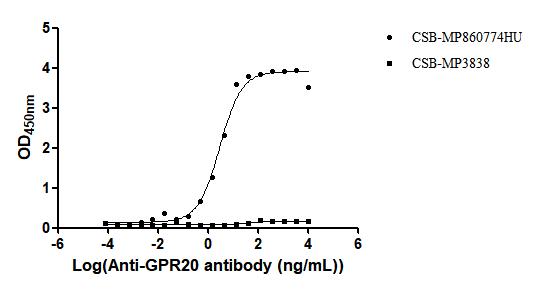



Comments
Leave a Comment