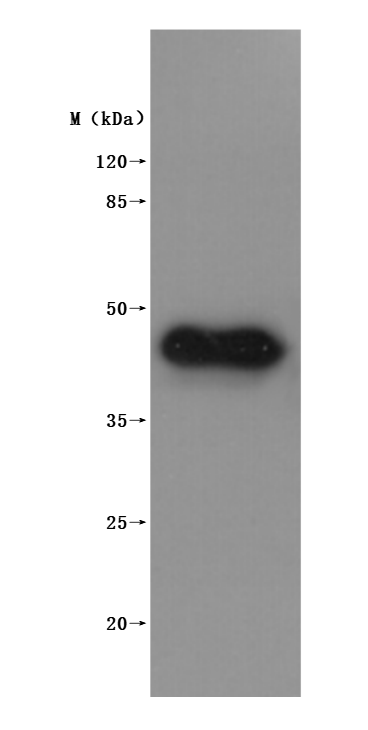
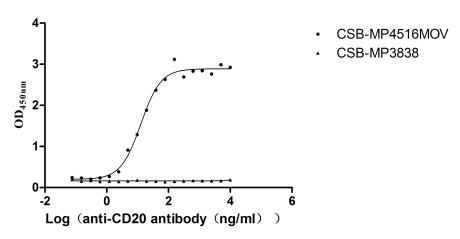
This virus-like particle (VLP) formulation of recombinant Macaca fascicularis MS4A1 (CD20) protein encompasses the full-length (amino acids 1-297) of MS4A1 protein, with a C-terminal 10×His tag. It is expressed in mammalian cells. The VLP architecture mimics native membrane protein conformation while exhibiting low endotoxin levels (<1.0 EU/μg, LAL method). Functional validation via ELISA demonstrates specific binding to anti-CD20 antibody (CSB-RA015007MA3HU) (EC50: 10.65–15.26 ng/mL at 10 μg/mL immobilization), with VLPs (CSB-MP3838) serving as negative controls to confirm binding specificity.
The mammalian expression system ensures proper post-translational modifications critical for maintaining MS4A1's structural epitopes. The VLP format enhances antigen avidity and preserves conformational epitopes, offering advantages over monomeric proteins for functional antibody characterization. This recombinant MS4A1 protein serves as a key reagent for advancing translational research in hematological malignancies and immunotherapeutic strategies.
The MS4A1 protein is a crucial component of the immune system, predominantly implicated in B-cell signaling and proliferation. In the context of Macaca fascicularis, or the cynomolgus monkey, MS4A1 has garnered attention due to its role in transplantation immunology and renal allograft rejection studies, as it is one of the relatively few B cell-specific genes expressed in non-human primates [1].
Recent advancements in the understanding of MS4A1 have highlighted its expression profile during certain immunological responses, which may parallel human responses. Smith et al. noted that RNA expression profiling of Macaca fascicularis renal allografts revealed MS4A1's involvement in determining graft survival and immune tolerance [1]. This relationship is further emphasized by the observation that the gene set includes numerous inflammation-related and NK cell-related genes, indicative of MS4A1's location within a complex network of immune response regulation in primates [1].
Additionally, since Macaca fascicularis shares approximately 92% genetic similarity with humans [2], studying this protein's immunological role in cynomolgus monkeys may provide valuable insights into human immunological processes, making them a pertinent model in biomedical research. Targeting the MS4A1 protein could potentially lead to novel therapeutic approaches to enhance transplant success and improve outcomes for patients requiring organ transplants, thus illuminating its relevance beyond just a primate model [1][2].
In terms of evolutionary biology, the MS4A1 gene is utilized in phylogenetic studies to understand the genetic relationships within the Macaca genus, particularly how such proteins have evolved to accommodate significant physiological and immunological demands across species [3]. This evolutionary aspect underscores the significance of MS4A1 in comparative studies that extend to understanding broader mammalian immune responses, notably to human health and disease mechanisms.
References:
[1] R. Smith, M. Matsunami, et al. Rna expression profiling of nonhuman primate renal allograft rejection identifies tolerance. American Journal of Transplantation, vol. 18, no. 6, p. 1328-1339, 2018. https://doi.org/10.1111/ajt.14637
[2] R. Smith, B. Adam, et al. Rna expression profiling of renal allografts in a nonhuman primate identifies variation in nk and endothelial gene expression. American Journal of Transplantation, vol. 18, no. 6, p. 1340-1350, 2018. https://doi.org/10.1111/ajt.14639
[3] B. Koo, D. Lee, et al. Reference values of hematological and biochemical parameters in young-adult cynomolgus monkey (macaca fascicularis) and rhesus monkey (macaca mulatta) anesthetized with ketamine hydrochloride. Laboratory Animal Research, vol. 35, no. 1, 2019. https://doi.org/10.1186/s42826-019-0006-0