The development of antibody-drug conjugates (ADCs) has revolutionized cancer therapy by enabling targeted delivery of cytotoxic agents to cancer cells. ADC's anti-cancer efficacy depends on its internalization efficiency. How to assess ADCs' internalization and the internalization efficiency is useful to identify appropriate antibody candidates with internalization characteristics favorable for ADCs.
Current methods for assessing antibody internalization are limited, often requiring complex assays and extended timelines. This bottleneck has hindered the rapid development of ADCs, highlighting the need for innovative solutions. The introduction of the DT3C protein offers a streamlined and efficient approach to this challenge, significantly accelerating the selection of promising ADC candidates.
Table of Contents
1. What is Antibody Internalization for ADC?
Antibody internalization is a critical mechanism in the function of ADCs, enabling the targeted delivery of cytotoxic payloads to cancer cells. This process involves the binding of the antibody component of the ADC to a specific antigen on the surface of the target cell, followed by the internalization of the antibody-antigen complex into the cell through receptor-mediated endocytosis. Once inside, the payload is released, leading to apoptosis by damaging DNA or inhibiting microtubules. It also kills nearby cancer cells through the bystander effect [1].
ADC efficacy depends on efficient antibody internalization, as many antibodies show poor uptake, leading to suboptimal payload delivery. Assessing internalization efficiently and accurately is essential for optimizing ADC efficacy.
Antibody internalization ensures that the cytotoxic payload is released in the lysosome, where it can exert its tumor-killing effects through DNA damage or microtubule inhibition [1]. Without efficient internalization, the payload would remain extracellular, reducing the ADC's therapeutic efficacy [1].
Internalization also helps reduce off-target toxicity, maximizing therapeutic specificity and minimizing adverse effects [2,3]. Internalization is also linked to the bystander effect, which is particularly beneficial in tumors with heterogeneous antigen expression [1].
The factors influencing the efficiency of internalization include different antibody isomer types, cell types, and the extracellular environment. Inefficient internalization could lead to the off-target release of ADC, resulting in systemic toxicity [4].
2. Current Antibody Internalization Assays
Antibody internalization assays are critical for understanding the mechanisms of immunotherapy resistance, particularly in cancer treatment. These assays often employ advanced techniques such as flow cytometry, fluorescent microscopy, and confocal microscopy, alongside cytotoxicity assays, to provide comprehensive insights into cellular uptake and its impact on cell viability.
Antibody Internalization Assessment Tools
| Methods |
Principles |
Features |
| Flow cytometry/ Fluorescent microscope |
Antibodies are labeled with pH-sensitive probes. These probes are non-fluorescent at neutral extracellular pH but emit fluorescence upon internalization into acidic endosomes/lysosomes (pH 4.5-6.0). Detection of fluorescence signal changes via flow cytometry. |
High specificity, Quick, Quantifiable |
| Confocal Microscopy |
Fluorescently labeled ADCs are co-localized with organelle-specific probes (e.g., LAMP1 for lysosomes). |
Real-time ADC observation, High resolution, kinetic monitoring |
| Cytotoxicity assays |
Toxins such as Mab-ZAP and DT3C are conjugated to antibodies. After internalization, the toxin is released, and internalization efficiency is indirectly evaluated by measuring resultant cytotoxicity. |
High-throughput; Primary and initial screening |
3. Recombinant Protein DT3C for Antibody Internalization
In 2014, MikiYamaguchi et al. produced a recombinant protein, DT3C, and confirmed that DT3C is superior to Mab–ZAP in screening antibodies internalized by cells [5]. DT3C lacks the receptor-binding domain, so that cells could not be killed in the absence of antibodies. DT3C was designed to form mAb–DT3C conjugates readily through the 3C domain, which would mimic the behavior of ADCs in vitro, providing a rapid and reliable method for identifying internalizing antibodies. The efficiency of mAb internalization by cells can be easily measured by monitoring what extent mAb-DT3C conjugates induce cell death.
To further investigate the role of DT3C in evaluating the internalization efficiency of ADC antibodies, Miki Yamaguchi et al. employed DT3C as a screening tool in 2015 to identify an anti-Mucin 13 monoclonal antibody (designated TCC56) for ADC designed for the treatment of pancreatic ductal adenocarcinoma cells [6]. They confirmed that TCC56‑DT3C conjugates induced cell death in TCC-PAN2 cells expressing MUC13.
According to available records, DT3C has been utilized in approximately 130 ADC monoclonal antibody screening patents as a dedicated tool for assessing the internalization capacity of mAbs.
DT3C is a recombinant protein engineered by removing the receptor-binding domain of diphtheria toxin (DT) and incorporating the C1, C2, and C3 domains of streptococcal protein G (3C). This modification allows DT3C to function as a cytotoxic agent when conjugated to a mAb, mimicking the action of an ADC in vitro.
The key advantage of DT3C lies in its ability to identify mAbs that are internalized by target cells. When a mAb-DT3C conjugate reduces the viability of cancer cells, it confirms that the mAb has been successfully internalized, making it a strong candidate for ADC development [5].
3.2 How Does DT3C Mediate the Detection of the Efficiency of mAbs' Internalization by Cells?
As previously noted, the internalization capability of antibodies is a critical factor in evaluating ADC drug efficacy. This raises two key questions: How does the internalization of ADC antibodies differ from that of naked antibodies? And why is DT3C considered a specialized tool for ADC analysis?
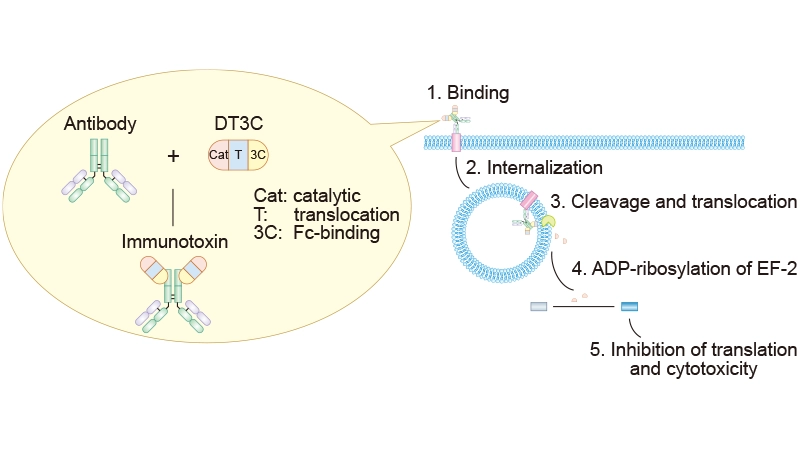
The internalization process indeed differs between ADC antibodies and naked antibodies. First, ADCs have a larger molecular weight, which affects their internalization efficiency. Second, DT3C conjugates with mAbs, forming an mAb–DT3C conjugate that mimics ADC behavior. As illustrated below, the mAb–DT3C complex binds to cell surface antigens and is internalized through endocytosis. Within the cytoplasm, DT3C is cleaved by furin protease, releasing its catalytic domain. This domain induces ADP-ribosylation of elongation factor 2 (EF-2), inhibiting protein synthesis and leading to cell death.
Figure 1. Mechanism of mAb-DT3C-induced cytotoxicity
3.3 The advantages of DT3C as a Tool for Evaluating ADC Antibody Internalization
Before DT3C, Mab-ZAP, a saporin-based immunotoxin, was the primary tool for assessing antibody internalization. While Mab-ZAP also induces apoptosis upon internalization, its large size and anti-mouse antibody components may reduce internalization efficiency. In contrast, mAb–DT3C conjugates are more compact and exhibit higher internalization efficiency, as supported by experimental data. Here are the advantages of DT3C as a tool for evaluating ADC antibody internalization.
① Simple Preparation: mAb–DT3C conjugates form after 30 minutes of incubation at room temperature and functionally mimic the effect of ADCs in vitro.
② Efficiency: The DT3C system simplifies the screening process by directly linking antibody internalization to a measurable outcome (cell viability) [5]. Internalization efficiency can be rapidly assessed via methods such as MTT, WST-1, or CTG assays.
③ Target-Dependent Toxicity: Cytotoxicity occurs only when mAb–DT3C is internalized by target antigen-expressing cells.
④ Broad Compatibility: DT3C binds to IgGs from various species (e.g., human, mouse, rabbit, goat), expanding its utility in internalization assays [5].
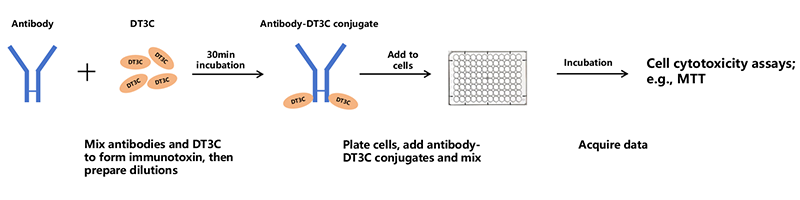
4. General Workflow to Assess Antibody Internalization with DT3C
Assessing antibody internalization is a critical step in understanding the efficacy and mechanism of action of therapeutic antibodies, particularly in the context of targeted cancer therapies. The DT3C protein is often used as a tool to evaluate internalization due to its ability to induce cell death upon internalization. Below is a detailed workflow to assess antibody internalization using the DT3C protein.
Figure 2. General workflow of assessing antibody internalization with DT3C
In summary, DT3C is a simple, efficient, and economical tool to evaluate the efficiency of antibody internalization during ADC development. Its ability to rapidly and efficiently assess antibody internalization addresses a critical bottleneck in ADC candidate selection. By leveraging this innovative tool, researchers can accelerate the discovery and optimization of ADCs, paving the way for more effective cancer therapies.
CUSABIO developed a recombinant DT3C (Diphtheria toxin&spg 3C domain) for the Antibody Internalization Assay. This DT3C protein can evaluate the internalization efficiency of the antibody in vitro. The mAb-DT3C complex preparation is simple. The DT3C is compatible with mAbs from various sources and demonstrates superior antibody internalization efficiency compared to Mab-ZAP.
High Purity Validated by SDS-PAGE
(Tris-Glycine gel) Discontinuous SDS-PAGE (reduced) with 5% enrichment gel and 15% separation gel.
Cell Cytotoxicity Experiment of DT3C
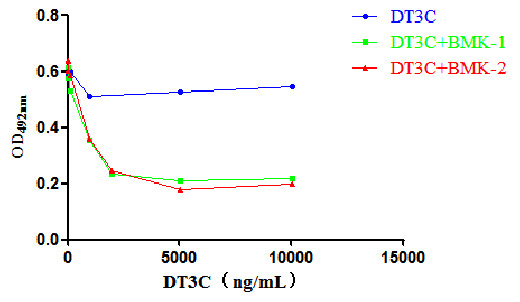
Binding of DT3C and CCR8 antibody. Upon cellular internalization, DT3C causes cell death and reduces cell viability. Therefore, the lower the number of surviving cells after internalization, the better the effectiveness of DT3C.
References
[1] Jin Y, Schladetsch MA, Huang X, Balunas MJ, Wiemer AJ. Stepping forward in antibody-drug conjugate development [J]. Pharmacol Ther. 2022 Jan;229:107917.
[2] Aoyama, M., Tada, M., Yokoo, H. et al. Fcγ Receptor-Dependent Internalization and Off-Target Cytotoxicity of Antibody-Drug Conjugate Aggregates [J]. Pharm Res 39, 89–103 (2022).
[3] Kleinman D, Iqbal S, et al. PLL-g-PEG Polymer Inhibits Antibody-Drug Conjugate Uptake into Human Corneal Epithelial Cells In Vitro [J]. J Ocul Pharmacol Ther. 2024 Sep;40(7):419-427.
[4] Fu, Z., Li, S., Han, S., Shi, C., & Zhang, Y. (2022). Antibody drug conjugate: The “biological missile” for targeted cancer therapy [J]. Signal Transduction and Targeted Therapy, 7, 93.
[5] Yamaguchi M, Nishii Y, et al. Development of a sensitive screening method for selecting monoclonal antibodies to be internalized by cells [J]. Biochem Biophys Res Commun. 2014 Nov 28;454(4):600-3.
[6] Nishii Y, Yamaguchi M, Kimura Y, et al. A newly developed anti-Mucin 13 monoclonal antibody targets pancreatic ductal adenocarcinoma cells [J]. Int J Oncol. 2015, 46(4):1781-7.
CUSABIO team. Streamlining Antibody Internalization Assessment with DT3C: Accelerating ADC Candidate Selection. https://www.cusabio.com/c-21065.html






Comments
Leave a Comment