2022 seems to be a "blowout" year in the field of bispecific antibody research and development. The number of drugs on the market in less than one year will exceed the sum of the number of bispecific antibody drugs on the market over the years. Bispecific antibody (BsAb) develops rapidly, the industry heat continues to rise, has become a hot spot in the field of many diseases treatment, such as cancer, inflammation, viral infection and autoimmune diseases.
According to the relevant database of clinical trains, there are currently more than 700 bispecific antibodies in the clinical and preclinical stages in the world. It is estimated that the bispecific antibodies market value will be as high as 80 billion dollars in 2030. In terms of indications, more than 80% of pipelines are concentrated in the field of tumor, and solid tumor racetrack is popular. In addition, BsAb is also expanding to the research field of inflammation, infection, eye disease and other diseases.
On October 25, 2022, Janssen, a unit of Johnson & Johnson, announced that the FDA approved TECVAYLI (telistamab cqyv) for the treatment of adult recurrent or refractory multiple myeloma. Previously, among the bispecific antibodies already on the market, the Hemlibra developed by Roche achieved sales of CHF 3.022 billion in 2021. Albeit a vast majority of bispecific antibodies agents are under clinical development, we believe that next years will witness clinical success of BsAbs!
1. What is a bispecific antibody?
Bispecific Antibodies (BsAbs), also known as bifunctional antibodies, are artificial antibodies containing two specific antigen binding sites. Technically, BsAbs are artificially engineered monoclonal antibodies that are capable of binding two different types of antigen or two different epitopes on the same antigen. BsAbs can be constructed by chemical cross-linkage, genetic engineering, or somatic hybridization. These methods generate mixtures of monospecific and bispecific antibodies, from which the desired bispecific molecule is achieved. Therefore, this dual specificity provides more possibilities in a more wide range of applications. Over the past decades, researchers and drugmakers have overcome many difficulties and challenges.
Seven BsAb drugs have been marketed worldwide, and five of them received FDA approval: Telistamab cqyv (2022, Tecvayli, Johnson & Johnson): targeting BCMA & CD3; Faricimab (2022, Vabysm, Genentech): targeting VEGF & Ang-2; Evantozumab (2021, Rybrevant, Johnson & Johnson): targeting EGFR & c-MET; Emicizumab (2017, Hemlibra, Roche): targeting FIX & FX; Beryntumomab (2014, Blincyto, Amgen): targeting CD3 & CD19; Catausomab (2009, Removab, 2017 withdrawn, Trion Pharma Co. Ltd.): targeting CD3 & EpCAM.
2. Bispecific Antibody Structure and Formats
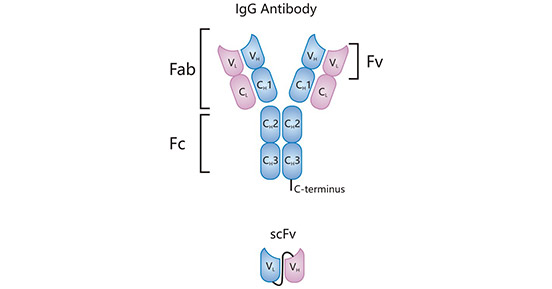
Natural antibody is a tetramer composed of two light chains (L) and two heavy chains (H), with a "Y" shape, containing two identical Fab domains and a Fc domain. IgG is the most abundant antibody in the blood and it is the backbone most often used for antibody therapeutics, the structure is shown in Figure 1. The formats of BsAb can generally be classified on the basis of structural features. There are two main categories of BsAbs: bispecific antibodies without Fc fragments (non-IgG-like bispecific antibodies) and bispecific antibodies with Fc fragments (IgG-like bispecific antibodies).
Figure 1. The IgG antibody construct consists of Fab and Fc regions. The binding part of the Fab region is called the single chain variable fragment (scFv). The antibody exists of two heavy chains (VH and CH) and two light chains (VL and CL). These chains can be subdivided by variable (VH and VL) and constant domains (CH and CL) [1]
● Non-IgG-like bispecific antibodies
Non-igG-like BsAb avoids strand cross-linking problems by using a fragmented molecular design that binds multiple antigen-binding units to molecules without Fc regions. Non-igG-like BsAb has the advantages of simple structure, strong tissue penetration, and low immunogenicity. However, due to their small molecular weight and lack of FcRn-mediated recycling mechanism, the half-life of drugs is generally short, and it is often necessary to use polyethylene glycol modification, albumin fusion, Fc fusion and other methods to improve the half-life of drugs.
● IgG-like bispecific antibodies
IgG-like BsAb retain Fc fragments and thus can exert Fc-mediated effector functions, such as antibody-dependent cell-mediated cytotoxicity, complement-dependent cytotoxicity(CDC), and antibody-dependent cell-mediated cytotoxicity(ADCC). It can be further divided into symmetric IgG-like BsAb and asymmetric IgG-like BsAb according to whether it is symmetric or not. IgG-like BsAb binds to the receptor FcRn through the Fc fragment and has a relatively longer serum half-life, which may be more advantageous in the frequency of administration.
3. How do bispecific antibodies work?
There are three main mechanisms of action of BsAbs: (1) mediated immune cell killing; (2) dual-target signal blockade; and(3) promote the formation of functional complexes.
● Mediated immune cell killing
An important mechanism of BsAbs is to mediate the killing of tumor cells by immune cells. BsAbs have two antigen-binding arms, one of which binds to the target antigen and the other to the marker antigen on the effector cells, which can activate the effector cells to target and kill tumor cells. T cells and NK cells are the most commonly used effector cell types, and the popular surface targets are CD3 and CD16.
● Dual-target signal blockade
In addition to direct killing of cancer cells, inhibition of cancer cell proliferation, invasion and metastasis is also an important part of cancer therapy. Receptor tyrosine kinases (RTKs) are the largest class of enzyme-linked receptors, which play an important regulatory role in the process of cell proliferation. Abnormal expression of RTKs on the surface of tumor cells can lead to malignant proliferation of tumor cells.
However, the receptors associated with cancer cell proliferation usually do not exist on the surface of tumor cells alone, but multiple receptors work together to maintain the proliferation of cancer cells. Therefore, the inhibition of cancer cell proliferation requires the simultaneous blocking of dual or even multiple targets, which can reduce the escape of tumor cells, overcome drug resistance, and improve the therapeutic effect.
● Promote the formation of functional complexes
The two antigen-binding arms of BsAbs can combine the characteristics of different antigens, and they bind to two specific protein molecules to form functional complexes. Administration of this complex can reduce the rejection reaction and improve the clinical therapeutic effect.
4. How are bispecific antibodies made?
BsAbs are primarily produced by three methods: (1) chemical conjugation, which involves chemical cross-linkers, (2) quadroma technology based on the somatic fusion of two different hybridoma cell lines, and (3) genetic approaches utilizing recombinant DNA technology.
● Chemical conjugation
The chemical conjugation method first appeared in the 1980s. Its principle is to couple two intact IgG or two F(ab)2 antibody fragments into a BsAb by chemical coupling agents (such as phthalimide, N-succinyl-3 - (2-pyridindithio) propionate, dithioacyl benzoic acid, etc.). Although this method is fast and simple, it is easy to destroy the antigen binding site and affect the activity of antibody. At the same time, the safety and carcinogenicity of the cross-linking agent itself are also uncertain.
● Somatic fusion of two hybridoma cell lines
Two different hybridoma cells were fused into double hybridoma cell lines by cell fusion, and then the target cells were cloned by conventional hybridoma screening method. Since the genetic background of double hybridoma is derived from the two hybridoma cells of the parent, it must produce two different heavy and light chains. The random association of these different heavy and light chains will lead to the mixing of antibodies, and it is necessary to purify and isolate BsAbs from the parental antibodies.
The preparation of BsAb by double hybridoma method is random and inefficient, but the biological activity of BsAb is relatively good and the antibody structure is stable. Konck-in-hole technique can effectively solve the problem of correct pairing of heterologous antibody heavy chains.
● Recombinant DNA technology
Wide adoption of genetic engineering facilitates today's exploitation of the huge potential inherent in bispecific antibodies: suitable polypeptide chains are designed in silico and expressed in various host systems, most frequently CHO cells and E. coli, followed by purification and assembly of the various components in vitro, resulting in multiple forms of bispecific antibodies.
5. Applications of Bispecific Antibody
BsAbs are widely used in diagnosis and treatment. In terms of diagnosis, BsAbs can not only be combined with horseradish peroxidase(HRPO) and used for pre targeting strategies to assist clinical diagnosis, but also provide better imaging examination for early detection, diagnosis and treatment of tumors. In terms of treatment, the main strategy is to precisely target and reactivate immune cells, help regulate the activation of immune cells, fine tune the fate and function of immune cells, improve the tolerance of immune cells, and promote the restoration of immune homeostasis. In addition to tumors, BsAbs can also treat other diseases, such as hemophilia A, diabetes, Alzheimer's disease and ophthalmic diseases.
6. Bispecific Antibody vs Monoclonal Antibody
Why are bispecific antibodies better? Compared with common antibodies, bispecific antibodies (BsAb) have more advantages since the basic BsAb construct is designed as "two connected units". Generally, monoclonal antibodies in combination have higher remission rates than single applied, but are more toxic. Surprisingly, dual antibodies are superior in efficacy and safety compared to single-antibody combination therapies.
Specifically, BsAb have more specificity and better performance in terms of T cell killing of tumor cells, off-target toxicity, and clinical indications that cannot be achieved with single-target antibodies. For example, a bispecific antibody used in cancer treatment, one binds to effector cells of molecules and the other binds to the tumor cells triggering lysis of target tumor cells.
Numerous clinical data have found that dual antibodies exhibit higher efficacy, better resistance, lower toxicity, and broader disease applicability compared to monoclonal or monoclonal antibodies in combination.
7. Popular Bispecific Antibody Targets in Clinical Trials
Overall, the targets of bispecific antibodies from China are mainly CD3, HER2, PD-1, PD-L1, EGFR, CTLA-4, etc., which is basically consistent with the distribution worldwide. Apparently, the favorite targets cover the immune targets, PD-1, PD-L1, BCMA, CD47, CTLA-4, LAG-3, 4 -1BB, and other popular research targets, CD19, CD20, CD33, CD38, HER2, VEGF, LAG-3, EGFR, Claudin 18.2, CEA, PSMA, RANKL, c-MET, DLL3, IL4, etc.
To fully serve researchers and pharmaceutical companies in the development of bispecific antibodies (BsAb), CUSABIO offers a wide range of proteins, which are the most popular targets to stimulate the BsAb research and development. (Click on the target names below to view more).
BsAb Popular Targets in Clinical Trials (China and Foreign)
Other BsAb Popular Targets in Clinical Trials (China)
Other BsAb Popular Targets in Clinical Trials (Foreign)
References
[1] Suurs, Frans V. et al. "A review of bispecific antibodies and antibody constructs in oncology and clinical challenges." Pharmacology & therapeutics 201 (2019): 103-119.
[2] Ma, Jiabing, et al. "Bispecific Antibodies: From Research to Clinical Application." Frontiers in Immunology 12 (2021): 1555.
[3] You, Gihoon, et al. "Bispecific Antibodies: A Smart Arsenal for Cancer Immunotherapies." Vaccines 9.7 (2021): 724.
[4] Weidanz, Jon. "Targeting cancer with bispecific antibodies." science 371.6533 (2021): 996-997.
[5] Anxin Securities Report-Biopharmaceutical industry innovative drug research of bispecific antibodies analysis: emerging technologies into the rapid development period. 2021.
CUSABIO team. Bispecific Antibody Overview: From Definition to Application. https://www.cusabio.com/c-21056.html




Comments
Leave a Comment