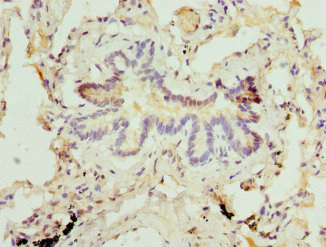
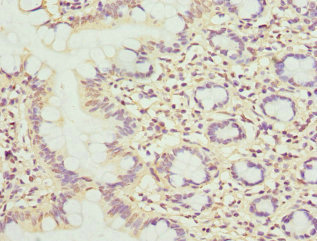
Serine protease that cleaves after hydrophobic or small residues, provided that Arg or Lys is in position P4: known substrates are SREBF1/SREBP1, SREBF2/SREBP2, BDNF, GNPTAB, ATF6 and ATF6B. Cleaves substrates after Arg-Ser-Val-Leu (SREBP2), Arg-His-Leu-Leu (ATF6), Arg-Gly-Leu-Thr (BDNF) and its own propeptide after Arg-Arg-Leu-Leu. Catalyzes the first step in the proteolytic activation of the sterol regulatory element-binding proteins (SREBPs) SREBF1/SREBP1 and SREBF2/SREBP2. Also mediates the first step in the proteolytic activation of the cyclic AMP-dependent transcription factor ATF-6 (ATF6 and ATF6B). Mediates the protein cleavage of GNPTAB into subunit alpha and beta, thereby participating in biogenesis of lysosomes. Involved in the regulation of M6P-dependent Golgi-to-lysosome trafficking of lysosomal enzymes. It is required for the activation of CREB3L2/BBF2H7, a transcriptional activator of MIA3/TANGO and other genes controlling mega vesicle formation. Therefore, it plays a key role in the regulation of mega vesicle-mediated collagen trafficking.