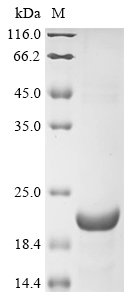
Recombinant Human 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (SRD5A2) is produced in E. coli and covers amino acids 29-71. This partial protein includes an N-terminal 6xHis-KSI tag to help with purification and detection. The product shows purity levels above 85%, confirmed through SDS-PAGE analysis, and is designed strictly for research purposes.
SRD5A2 appears to be a vital enzyme in steroid metabolism. It catalyzes the conversion of testosterone to dihydrotestosterone (DHT), which is a more potent androgen. The enzyme likely plays an important role in developing and maintaining male characteristics and participates in hormonal regulation pathways. Studies of SRD5A2 may provide valuable insights into androgen-related processes and various conditions.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
The expressed region (29-71aa) represents only 43 amino acids, which should have a theoretical molecular weight of approximately 4.7 kDa. The large discrepancy with the measured 20.0 kDa confirms the substantial size of the N-terminal 6xHis-KSI fusion tag, which dominates the protein construct. This extremely short functional fragment (only 18% of the full-length protein) coupled with a large fusion tag makes proper folding of any potential SRD5A2 domain highly unlikely. The protein is essentially a fusion tag with a very small SRD5A2 fragment attached, incapable of adopting the native structure required for enzymatic activity. The >85% purity indicates a clean preparation but does not validate structural integrity of the SRD5A2 portion.
1. Antibody Development and Epitope Mapping
This application remains feasible but with limitations. The recombinant SRD5A2 protein can generate antibodies against the 29-71aa linear sequence. However, the dominant fusion tag may cause most antibodies to recognize tag epitopes rather than the small SRD5A2 fragment. The 20.0 kDa size confirms the fusion tag dominates the construct. Resulting antibodies require rigorous validation against full-length SRD5A2 to ensure specificity.
2. ELISA-Based Binding Assays
This application is only suitable for tag detection, not SRD5A2 functional studies. The 20.0 kDa size confirms the protein is primarily fusion tag, making it useful for anti-His or anti-KSI antibody validation but irrelevant for studying SRD5A2-specific binding properties.
3. Biochemical Characterization and Stability Studies
This application can characterize the fusion protein construct but provides no meaningful information about native SRD5A2. Studies will reflect properties of the dominant KSI tag with minimal contribution from the small SRD5A2 fragment.
Final Recommendation & Action Plan
The confirmed 20.0 kDa molecular weight fundamentally changes the assessment of this SRD5A2 protein's utility. The construct is predominantly fusion tag (~75% of the mass) with only a minimal SRD5A2 fragment. Recommended actions: 1) Use exclusively for generating antibodies against the 29-71aa linear epitope, with thorough validation against full-length SRD5A2; 2) Completely avoid all functional studies including interaction assays, as results will reflect tag artifacts; 3) For legitimate SRD5A2 research, obtain full-length protein without dominant fusion tags.