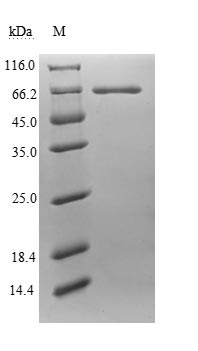
N-terminal 6xHis-tagged recombinant human Endogenous retrovirus group K member 6 Env polyprotein (ERVK-6) is a partial-length protein containing amino acid 90-632. It is the extracellular domain of human ERVK-6 protein and can be used for the production of corresponding specific antibodies. Procaryotic expression (E.coli) confers this recombinant ERVK-6 good features such as clear genetic background and relatively simple separation & purification. The purity of ERVK-6 protein is greater than 85% determined by SDS-PAGE. This recombinant ERVK-6 protein may also be used in the studies of microbiology.
Human ERVK-6 is a member of human endogenous retrovirus type K (HERV-K), the sole group of endogenous retroviruses that are established to contain human-specific members. As a retroviral envelope protein, ERVK-6 mediates receptor recognition and membrane fusion during early infection. This endogenous envelope protein has lost its original fusogenic properties during evolution.